Abstract
Isothermal information is rarely available for the formation of martensite in Fe or Fe alloys due to a very high rate of transformation compared to the rate of heat conduction. Such information has now been extracted for lath martensite in some sets of Fe alloys from available information on ultra-rapid quenching but only at a single temperature for each alloy, related to its two MS temperatures. The temperature dependence could, thus, be studied only on binary sets of alloys. Those results have been applied to mathematical models based on the Arrhenius equation and illustrated with Arrhenius plots. For three sets of binary Fe alloys, a large group of rates came close to the rate of an almost pure and carbon-free Fe-C alloy. It illustrated that Cr, Ni, and Ru in low contents have relatively small effects on the rate of formation of lath martensite in Fe. It also demonstrated that the present measurements have considerable reproducibility. In contrast, a set of Fe-C alloys did not give a straight line in the Arrhenius plot. Using a new mathematical model based on the concept of the Arrhenius equation to express the effect of carbon, it was possible to predict the rate of formation of lath martensite for Fe-C alloys with fixed C content and their temperature dependencies which are not available experimentally due to the very high rate of formation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
It is very difficult to measure the rate of formation of martensite in Fe alloys due to the very high rate of formation. Thus, isothermal information on martensite at specified temperatures is particularly difficult to obtain for the formation of various kinds of martensite in Fe alloys due to the very high rate of formation, compared to the rate of heat conduction. Exceptions have been classified as isothermal martensite.[1,2,3,4] A Russian group[5,6,7,8,9,10,11,12] instead applied ultra-rapid quenching on steels that could then form both lath and plate martensite. They found that the formation of lath martensite can be suppressed by very high cooling rates, and the rate of formation should, thus, be limited although at a very high level. They determined the critical cooling rate for suppression, \(v^{\prime\prime}_{cr}\), of 30 Fe-alloys and concluded that lath martensite may, thus, be compared to the exceptional case of isothermal martensite in the sense that both take some measurable time to form, which may be used as a measure of the rate of formation. Their conclusion was accepted in the present work, and it was decided to try to extract more detailed information on the rate of formation of lath martensite from their information. It will be shown how the temperature dependence can be represented by an activation energy, and the relation to slow isothermal martensite will be demonstrated by applying modeling that may apply to both slow isothermal martensite and rapid lath martensite. One model is intended for substitutional alloying elements and another one for carbon.
2 Critical Time for Complete Formation of Lath Martensite
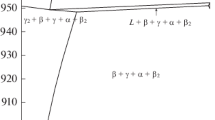
The primary results from the ultra-rapid quenching experiments were published as graphs, exemplified by the reproduction in Figure 1 for some Fe-C alloys.[5]
Determination of two MS temperatures of Fe-C alloys at various cooling rates, as reported by Mirzayev et al.[5] Three low C alloys: \({\text{M}}_{{\text{s}}}^{{\text{L}}}\) (upper lines) and \({\text{M}}_{{\text{s}}}^{{\text{P}}}\) (lower lines). Two high C alloys: Only \(M_{s}^{P}\) lines. Reprinted with permission from Ref. [5]
For the three low C alloys in Figure 1, it is evident that lath martensite did not have sufficient time to form a detectable amount above the \({\text{M}}_{{\text{s}}}^{{\text{P}}}\) line during cooling at a rate above a critical cooling rate, \(v^{\prime}_{cr}\), which was the basis of the conclusion of similarity to isothermal martensite. However, there was another critical cooling rate, \(v^{\prime}_{cr}\), below which plate martensite cannot form. It may be concluded that lath martensite has then had enough time to complete the martensitic transformation during cooling through the temperature range between the \({\text{M}}_{{\text{s}}}^{{\text{L}}}\) and \({\text{M}}_{{\text{s}}}^{{\text{P}}}\) temperatures. It is now proposed that this critical time can be estimated directly from the critical cooling rate, \(v^{\prime}_{cr}\), as follows:
It is further proposed that the inverse of the critical time, 1/tcr, can be used as a measure of the rate, V, of formation of lath martensite in units of s−1 or fraction per second, which means a complete transformation of the alloy in tcr seconds. The same unit was used by Villa et al.[4] in their recent study of the rate of formation of isothermal martensite.
The two kinds of critical cooling rates were evaluated from similar graphs for the remaining 30 binary or ternary Fe alloys,[5,6,7,8,9,10,11,12] utilizing the first experimental point on the \({\text{M}}_{{\text{s}}}^{{\text{P}}}\) line for \(v^{\prime}_{cr}\) and the last experimental point on the \({\text{M}}_{{\text{s}}}^{{\text{L}}}\) line for \(v^{\prime\prime}_{cr}\). The values were read off a scanned copy of Figure 1 and the other graphs using the Plotdigitizer software. The critical times are presented in Appendix Table A1 together with the two MS temperatures and the two critical cooling rates. The values of \(v^{\prime}_{cr}\) will be examined in detail in the following, and it would be of interest to estimate their errors. The primary uncertainty concerns the cooling rate in the primary experiments, but those authors did not give such information. The uncertainty of the present estimate of \(v^{\prime}_{cr}\) from the graphs depends primarily on the distances between the experimental points in the graphs. They vary considerably between the alloys and irregularly for each alloy. It was decided that the most objective method would be to use the first points and to accept that there could certainly be a considerable systematic error. In fact, there will also be a systematic error in the estimate of the temperature of formation. On the other hand, systematic errors may not hide a general dependence on temperature which is here of main interest. The present information on a rate process was, thus, applied to the Arrhenius equation in an attempt to demonstrate a temperature dependence.
For thermally activated rate processes, a constant activation energy (Q) is typically expected across a range of temperatures, supported by the linear relationship between the natural logarithm of the rate (V) and the reciprocal of temperature (1/T) as described by the Arrhenius equation. This equation is applied in the present study which will be based on the inverse critical time, 1/tcr, from Eq. [1] as a measure of the rate, V, of completing the transformation to lath martensite during very rapid cooling through the interval between the two Ms temperatures. The variable temperature in the interval will be approximated by an “effective” temperature, Teff, as presented in the following section, and there can be only one such value for each alloy. Consequently, this kind of rate has no temperature dependence for a single alloy, but one can evaluate the dependence of the rate on the Teff temperature for a group of alloys from a binary system. This Teff temperature will vary with the content of a solute element X, e.g., expressed as \(w_{x}\) mass pct, and one could regard the rate as a function of 1/Teff or \(w_{x}\). Experimentally, \(w_{x}\) is the independent variable that is chosen in advance of each measurement, and, in principle, the Teff temperature is then a dependent variable, being fixed through the Ms values of the alloy. The plots (Figure 1), thus, indicate that martensite formation information is only available for a single temperature for each individual alloy. However, a temperature-dependent trend becomes evident when comparing across various iron alloys, such as those in the studied Fe-C series.
It should be emphasized that for Fe-C alloys, this kind of information can be available only in the limited ranges of C content and temperature, which form the triangular area between the \({\text{M}}_{{\text{s}}}^{{\text{L}}}\) and \({\text{M}}_{{\text{s}}}^{{\text{P}}}\) lines in part of the Fe-C phase diagram. Already Mirzayev et al.[5] constructed this type of diagram from their experimental values, and it is reproduced in Figure 2. The upper limit of C content for such information should, thus, be about 0.65 mass pct C.
3 Effective Temperature of Formation Of Lath Martensite
Equation [1] suggests a linear relationship between the formation of lath martensite and temperature. However, for the purposes of the current study, the simple linear approximation provided by Eq. [1] is considered adequate for predicting the time required for complete formation. This is because the time to reach a complete martensitic transformation is primarily governed by the starting temperature, \({\text{M}}_{{\text{s}}}^{{\text{L}}}\), and finishing temperature, \({\text{M}}_{{\text{s}}}^{{\text{P}}}\), as well as the presented cooling rate, rather than the specific path taken between the two temperatures. It is important to realize, however that a non-linear model might more accurately capture the complex relationship between lath martensite fractions and temperature.
The fraction of lath martensite is expected to grow gradually during cooling between the two MS temperatures and the rate of formation is expected also to vary gradually with temperature. For the critical cooling rate, the formation of lath martensite is expected to be completed when the \({\text{M}}_{{\text{s}}}^{{\text{P}}}\) temperature is reached. This whole process was simply approximated by a hypothesized isothermal process at some effective temperature, Teff, and lasting the critical time, tcr, as obtained from Eq. [1]. It is not possible to predict the value of Teff without arbitrary assumptions and, for simplicity, it was instead approximated with the average temperature, Tav, of the transformation range.
4 Modeling of the Rate of Formation
Information on rates of formation is usually rationalized by applying the Arrhenius equation in Eq. [3][13] with two parameters that are independent of temperature which requires that one uses identical solution or material at all temperatures.
E is often regarded as an activation energy and Vo as a frequency factor. From the ultra-rapid cooling experiments, it was possible to extract information on the rate of formation of lath martensite but only at a single temperature for each alloy. To obtain any information on the temperature dependence of the rate of formation of lath martensite, it would be necessary to vary the transformation temperature by varying the alloy content in a set of binary Fe alloys which would certainly involve effects of the second element on the rate. Even for such cases, it was now decided to rationalize the temperature dependence with models based on the Arrhenius equation[13] in Eq. [3], which is widely accepted for reactions in solutions but also often modified to account for deviations.[14]
The rate V will always be expressed in some unit, convenient for the current situation and fraction per second in the present case. In any application of several rate measurements from a series of temperatures, the values of the two constant parameters will be determined simultaneously by optimizing the representation of the experimental information. Eq. [3] can conveniently be expressed logarithmically to be regarded as a function of 1/T.
On the other hand, Arrhenius presumed that the lnVo parameter was constant and it was then eliminated when he considered the ratio between Eq. [3] from any two temperatures. The E parameter could then be calculated for each such pair of temperatures, and they should all give the same E value if the equation was adequate for the case considered. The same procedure can be applied by using Eq. [4] and considering the difference between two temperatures.
The lnVo parameter is again eliminated if it is constant. It is evident that an E value can here be obtained directly for any combination of information from two temperatures and with the same E value for all combinations unless the lnVo parameter is not constant. When several measurements are inserted in Eq. [5] instead of Eqs. [3] or [4], then an optimization could be improved only by adjusting the E parameter and the result will not be identical to the one after optimization of the representation of the same information from Eqs. [3] or [4] with two adjustable parameters. In the present work, this difference will be emphasized by a special symbol, Q instead of E, with a basic definition as the derivative in Eq. [6].
Equations [4] and [5] will now be applied on the (1/tcr, Tav) information for the alloys in Appendix Table A1.
It should be emphasized that the models used in the current study were strictly based on mathematical merits. While it is acknowledged that a more comprehensive model integrating physical principles would offer advantages, such an approach was beyond the scope of our current research objectives.
5 Modeling of Fe-M Alloys
All entries in Appendix Table A1 are Fe alloys and are arranged in sets of binary or ternary alloys of similar components. The Fe-C set contained one alloy with only 0.004 mass pct C. It was added to the other sets to play the role of pure Fe. To be recognized in the figures, it will be shown as an open circle. The (1/tcr, Tav) information on all alloys in the three sets of Fe-Cu, Fe-Ni, and Fe-Ru alloys was first inserted as (V,T) pairs in Eq. [4]. The E and lnVo parameters are required to be constant in Eq.4 and fitted to each set of data resulting in the lnV(1/T) functions shown as straight lines in the Arrhenius plots in Figure 3 along with the root-mean-square error (RMSE) of the optimizations.
Arrhenius plots based on Eq. [4], when applied to the rate of formation of lath martensite in binary Fe alloys with Cu, Ni, or Ru. Lines represent slopes of –E/R
Equation [5] can also be applied here by selecting pure Fe, with lnV = 7.29 and Tav = 753 K, as alloy number 1 giving Eq. [7].
The information on the Fe-M alloys was inserted into Eq. [7] and the Q parameters were fitted to the information from the three sets. With the Q values inserted in Eq. [7], it would act as the lnV(1/T) functions for the three binary sets. As evident from Eq. [5], all the predicted lines using Eq. [7] must start from the point for pure Fe as illustrated in Figure 4.
Except for the starting point, the results of Eqs. [4] and [7], as displayed in Figures 3 and 4, are very similar, and it is shown that both Eqs. [4] and [7] represent the information rather well. The fact that the fitting was performed with only one adjustable parameter in Figure 4, Q, did not seem to matter much, probably because lnVo was already rather constant when calculated by inserting the Q value in Eq. [4] for all alloys in each set.
All data points in Figures 3 and 4 fall close to the same level as pure Fe, and it may seem evident that the second element, Cu, Ni, and Ru, have relatively weak effects on the rate. On the other hand, the second element has decreased the temperature of formation which by itself should have decreased the rate of formation of Fe with a slope of − E/R according to Eq. [4], where E should ideally be a constant, conveniently denoted as EFe. It would seem that the three second elements have happened to have an opposite effect and give a low over-all effect on the rates. The present information does not give any indication of how to separate the two effects to account for the presence of a second element. The fact that the many data points could form a thin band indicates that the present method to extract information of the temperature dependence for the rate of formation of lath martensite from ultra-rapid quenching deserves some confidence.
6 Modeling of Fe-C Alloys
There are five binary Fe-C alloys in Appendix Table A1 and their (1/tcr; Tav) pairs of values were inserted in fitted to both Eqs. [4] and [7] and presented as Arrhenius plots in Figure 5.
It is evident that these equations are not adequate for the Fe-C information since the experimental rates cannot support any straight line. There may rather be indications of a curved line which might be caused by the presence of C as a second element.
Formally, one could introduce the C content as wC mass pct as an extra variable in an equation for Fe-C alloys. However, each alloy has only one wC value and one Tav value and it is evident that wC in mass pct and Tav in kelvin are mutually dependent variables. Their relation is obtained from those values for Fe-C alloys in Appendix Table A1 that are compiled in Figure 6.
Experimental information from Appendix Table A1 on the relation between the C content, \({\text{w}}_{{\text{C}}}\), and the 1/Tav variable of lath martensite in Fe-C alloys
The information on Fe-C alloys in Figure 6 can be well represented by a straight line, defining the kC coefficient in Eq. [8]:
\({\text{T}}_{{{\text{Fe}}}}^{{{\text{av}}}}\) is the Tav value for the formation of lath martensite in pure Fe, which was already approximated by the value 753 K from the Fe-C alloy with only 0.004 mass pct C in Appendix Table A1. kC = d(wC)/d(1/Tav) = 1500 mass pct K−1 but the \(w_{C}\) variable and the ordinary T for isothermal martensite are independent variables. It seems possible to predict a curved V line depending on both 1/T and wC by fitting a modified equation to the present information and is presented in the following section.
7 Modified Model for Fe-C
When modeling the effect of dissolved elements in Fe, it seemed natural to let them act on the parameters that are already present in the Arrhenius equation where they are kept constant, Vo and E in Eq. [3]. Substitutional solutes were assumed to mainly affect the energy of the lattice, i.e., through the E parameter, because those atoms replace Fe atoms in their lattice sites and should change the properties of the Fe lattice that are important during the martensitic transformation. The direct effect of carbon atoms on the E parameter was, thus, neglected and, for clarity, it was denoted as EFe and is still assumed to be independent of temperature. Their effect on the Vo parameter was modeled by dividing the value of \({\text{V}}_{{\text{o}}}^{{{\text{Fe}}}}\) for pure Fe with a (\(1 + {\text{q}}_{{\text{C}}} {\text{w}}_{{\text{C}}}\)) factor. Except for that, both parameters in Eq. [3], now denoted as EFe and \({\text{V}}_{{\text{o}}}^{{{\text{Fe}}}}\), are still treated as independent of temperature. With this modification, Eq. [3] is presented as follows:
for Fe-C alloys at any given temperature, T, under isothermal conditions and valid for hypothetical isothermal martensite. There are three parameters, \({\text{q}}_{{\text{C}}}\), ln \({\text{V}}_{{\text{o}}}^{{{\text{Fe}}}}\) and EFe, but with one relation:
obtained by applying the information accepted for pure Fe from an alloy in Appendix Table A1. The ln \({\text{V}}_{{\text{o}}}^{{{\text{Fe}}}}\) parameter in Eq. [10] was then eliminated to yield
for hypothetical isothermal martensite. This model was also applied to lath martensite with a single Tav temperature for each alloy. However, it may then be necessary to accept the strict relation between the two variables, \({\text{w}}_{{\text{C}}}\) and Tav, represented by Eq. [8]. This was imposed on Eq. [12] to yield
for lath martensite. It should be noted that two different variable superscripts are used to differentiate between the two conditions: VFe-C denotes lath martensite and VFeC denotes isothermal martensite. Information on the Fe-C alloys with lath martensite was now inserted in Eq. [13], and the parameters were fitted to the information, yielding \({\text{q}}_{{\text{C}}}\) = 72 and EFe = − 65 kJ. The resulting Arrhenius plot for lath martensite is presented in Figure 7 together with the straight line from the Eq. [7] in Figure 5.
It may be objected that an activation energy should never be negative. However, in the Arrhenius equation, it is stipulated that the two parameters are independent of temperature. That is not satisfied in the present case and EFe just represents a parameter in earlier, here called the modified model, even though it seems to represent pure Fe.
The predicted Arrhenius plot for lath martensite in Figure 7 shows a reasonably good fit of the experimental information. The same parameter values can be inserted in Eq. [12] to act as model for hypothetical isothermal martensite of the same material. It is particularly interesting to compare the predicted Q quantities for the two kinds of martensite on the same material. For isothermal martensite, \({\text{Q}}^{{{\text{FeC}}}}\) is obtained as follows:
from a partial derivative of Eq. [12] under constant C content. For lath martensite, QFe-C is obtained as a partial derivative of Eq. [13].
Equation [15] contains the \(k_{C}\) coefficient from Eq. [8] instead of the \(w_{C}\) content in Eq. [14]. The \(k_{C}\) coefficient was given the same position as \(w_{C}\) in Eq. [14] in order to indicate that the content of C is given by the \(k_{C}\) coefficient.
Using Eq. [14] as a model for hypothetical isothermal martensite, one could relate the unknown QFeC to QFe-C by accepting the optimized \(q_{C}\) value. Combination of Eqs. [14] and [15] yields
It has, thus, been possible to get an idea about the temperature dependence of the rate for transforming hypothetical isothermal martensite Fe-C alloys from information on ultra-rapid cooling experiments of a set of Fe-C alloys, transforming to lath martensite. The difference between the two conditions can be illustrated by a 3D picture in Figure 8 of the lnVFeC(1/T, \({\text{w}}_{{\text{C}}}\)) function for hypothetical isothermal martensite in Eq. [14] with the parameters obtained from Eq. [15] for lath martensite.
The lnVFeC surface in Figure 8 could be covered by parallel lines for a series of constant \({\text{w}}_{{\text{C}}}\) values but only two are outlined. On the base plane, there is the straight line for the set of Fe-C alloys from Figure 6, and its projection on the lnVFeC surface is shown because the two martensites are supposed to come from the same material and should, thus, have the same rates. The projected line is identical to the curve already presented in Figure 7. The Q quantities for the two martensites are represented by the slopes of the two kinds of lines on the VFeC surface. They are indicated by small arrows and illustrate how it is possible that the two martensites have different Q values although they have the same rate properties and move on the same lnV surface when the temperature changes.
8 Discussion
This work deals with the rate of formation of the lath martensite that has formed gradually between two MS temperatures, during very rapid quenching where no isothermal observations can be made. It was reported that the formation of lath martensite could be suppressed by a sufficiently high cooling rate which indicated that there is a limited rate for the formation of lath martensite. The rate of formation of lath martensite was evaluated from the time spent between the two MS temperatures when the lath martensite formation has had time to be completed. For 30 alloys, it was succeeded to extract the critical time required for completion of the formation of lath martensite between the two MS temperatures, see Appendix Table A1. The inverted critical time was accepted as a measure of the rate, V, of formation of lath martensite in the unit of fraction per second. This information was limited to one measurement for each alloy and in order to study the effect of temperature on the rate of formation of lath martensite, it was necessary to vary the temperature by varying the alloy content.
It is not self-evident that these experimental results will be quite reliable. In addition, the temperature of formation could only be estimated as a so-called effective temperature, which was simply taken as the middle of the temperature range between the two MS temperatures. On the other hand, the main hope was to evaluate the temperature dependence of the formation and systematic errors, common to all rates, may not be too severe for that purpose when one evaluates temperature differences. The final test of the credibility would be whether or not the information would stand a close kinetic examination by producing sensible results by modeling. That seems to have been the case and, in addition, it was particularly satisfying that the reproducibility was sufficient to yield a reasonably thin scatter band around the rate for pure Fe for the Fe-M alloys in Figures 3 and 4.
For carbon, as a second element, there are strong indications of nonlinearity. To fit the information with the same type of model, it must be modified by accounting for the effect of carbon specifically. That was done by introducing an lnV(1/T,wC) function, where wC is mass pct C. It had two constant but adjustable parameters that were evaluated to give the best fitting of the model to the information from the present Fe-C alloys, Figure 7.
It may be emphasized that the information on lath martensite, obtained from very rapid quenching, has here been accepted to be identical to the expected information from a not available isothermal technique at the same temperatures but containing only a minor subunit of it. It may be regretted that the present information on Fe-C alloys is so meager, but it should be remembered that the present information covers the available range of carbon contents which was explained to be limited to a triangle in Figure 2 and two third of the range of carbon is already covered.
It should be realized that models have been applied in the present work in order to test an unusual kind of information. It may be stressed that the model, which was applied to represent the effect of carbon on the rate of formation of lath martensite, was selected on strictly mathematical merits. It seems to be a good choice because it was able to demonstrate that an Arrhenius plot with an extreme deviation from the usual straight line could be described by a relatively simple model. On a 3D picture of the lnV(1/T,wC) function, Figure 8, it was possible to demonstrate that the experimental methods can be represented by different lines on the lnV(1/T,wC) surface. In the present case, one kind of line is straight and the other very curved. It was even able to demonstrate how the temperature dependence of the rate could differ for different partial derivatives of the same function for the rate of formation. This result contributes to the credibility of the unusual kind of information.
9 Conclusions
The conclusions are as follows:
-
1.
The rate of formation of lath martensite in Fe alloys can be evaluated from information on ultra-rapid cooling experiments, giving a critical rate above which lath martensite cannot form due to an even higher rate of formation of plate martensite. However, this applies only to a special temperature for each alloy which, for convenience, was defined as the average between the two MS temperatures, Tav.
-
2.
The Arrhenius equation with its two parameters, Vo, and E, can be applied directly to a number of measurements or by first eliminating Vo, which Arrhenius did originally. This makes a difference only if the equation is modified by allowing one or both of the parameters to vary.
-
3.
Cr, Ni, and Ru in low contents have relatively small effects on the rate of formation of lath martensite in Fe.
-
4.
The Q or E values for all the substitutional Fe alloys were found to be rather similar and independent of temperature. This can hardly be caused by random scatter, compensating for actual differences. It is concluded that experimental scatter is limited. This is also supported by the impression of a smooth, curved line, obtained for Fe-C alloys. It would have been hidden by a stronger scatter.
-
5.
It follows that the lines in Arrhenius plots are fairly straight for the substitutional Fe alloys which indicates that the two parameters are rather constant whereas the strong curvature for the interstitial Fe-C alloys reveals strong variation. This justifies a strictly mathematical model based on the Arrhenius equation.
-
6.
Using a new mathematical model based on the concept of the Arrhenius equation to express the effect of carbon, the rate of formation of lath martensite for Fe-C alloys with fixed C content and their temperature dependencies which are not available experimentally due to the very high rate of formation can be predicted.
-
7.
It is illustrated how lath martensite and hypothetical isothermal martensite have different Q values although they have the same rate properties and move on the same lnV surface when the temperature changes.
References
G.V. Kurdjumov and O.P. Maksimova: Dokl. Akad. Nauk SSSR, 1948, vol. 61, pp. 83–6.
A. Borgenstam and M. Hillert: Acta Mater., 1997, vol. 45, pp. 651–62.
A. Borgenstam and M. Hillert: Acta Mater., 2000, vol. 48, pp. 2777–85.
M. Villa and M.A.J. Somers: Scr. Mater., 2018, vol. 142, pp. 46–49.
O.P. Morozov, D.A. Mirzayev, and M.M. Shteynberg: Phys. Met. Metallogr., 1972, vol. 34, pp. 114–19.
D.A. Mirzayev, O.P. Morozov, and M.M. Shteynberg: Phys. Met. Metallogr., 1973, vol. 36, pp. 99–105.
M.M. Shteynberg, D.A. Mirzayev, and T.N. Ponomareva: Phys. Met. Metallogr., 1977, vol. 43, pp. 143–49.
D.A. Mirzayev, M.M. Shteynberg, T.N. Ponomareva, and V.M. Schastlivtsev: Fiz. Met. I Metalloved., 1979, vol. 47, pp. 985–92.
D.A. Mirzayev, M.M. Shteynberg, T.N. Ponomareva, and V.M. Schastlivtsev: Phys. Met. Metallogr., 1980, vol. 47, pp. 102–11.
D.A. Mirzayev, M.M. Shteynberg, T.N. Ponomareva, B.Y. Bylskiy, and S.Y. Karzunov: Phys. Met. Metallogr., 1981, vol. 51, pp. 116–27.
D.A. Mirzayev, S.Y.E. Karzunov, V.M. Schastlivtsev, I.L. Yakovleva, and Y.E.V. Kharitonova: Phys. Met. Metallogr., 1986, vol. 62, pp. 100–109.
D.A. Mirzayev, V.N. Karzunov, V.N. Schastlivtsev, I.I. Yakovleva, and Y.V. Kharitonova: Phys. Met. Metallogr., 1986, vol. 61, pp. 114–22.
S. Arrhenius: Zeitschrift Für Phys Chemie, 1889, vol. 4U, pp. 226–48.
J.R. Hulett: Q. Rev. Chem. Soc., 1964, vol. 18, pp. 227–42.
Acknowledgments
This work was performed within the Competence Centre Hero-m 2i financed by VINNOVA (the Swedish Governmental Agency for Innovation Systems), Swedish industry, and KTH Royal Institute of Technology.
Funding
Open access funding provided by Royal Institute of Technology.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix
Appendix
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kumpati, J., Hillert, M. & Borgenstam, A. Evaluation and Modeling of the Rate of Formation of Lath Martensite in Fe-C Alloys, Extracted from Ultra-Rapid Quenching Experiments. Metall Mater Trans A 55, 2913–2921 (2024). https://doi.org/10.1007/s11661-024-07445-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11661-024-07445-1