Abstract
The paper deals in depth with characterizing bead-on-plate welds on EN-GJS-500-14 base metal, utilizing two filler metals: a pure Ni wire and a NiFe wire containing 45 wt pct Ni. The welds were conducted using the same heat input to ensure comparability in microstructure analysis. The microstructural observations were carried out using optical and scanning electron microscopies, X-ray tomography, X-ray diffraction analysis, and microhardness testing. Thermodynamic simulations using the non-equilibrium Scheil solidification model provide insights into the solidification process and the underlying metallurgical factors associated with the observed microstructural evolution. The observations revealed that the pure Ni wire deposited a softer fusion zone with graphite precipitation, while cementite precipitated in the fusion zone of NiFe alloy. The formation of martensite structures with different morphologies was the predominant microstructural evolution in the heat-affected zone of both welds. The partially melted zone of the pure Ni weld is narrower than the NiFe weld because more diffusion of Ni avoids widening the brittle structures in the partially melted zone. An unmixed zone in the form of a peninsula was exclusively observed in the fusion zone of the NiFe weld because of inadequate diffusion of Ni into the liquified materials along the fusion boundary.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Ductile cast iron (DCI) is widely used in various industrial components of wind turbines, such as hubs, shafts, gearbox housings, bed frames, etc., due to its versatility and high performance with high strength, ductility, toughness, and at the same time, easy to cast and low cost.[1,2,3] The microstructure of DCI is characterized by spheroidal graphite nodules dispersed throughout a matrix.[4] In general, the welding of DCI can be challenging, most notably because of the high carbon content, which leads to the formation of hard and brittle structures in the weld, resulting in a loss of ductility and a greater propensity for cracking.[5,6]
During the heating cycle of welding DCI, the region adjacent to the fusion line undergoes partial melting, known as the partially melted zone (PMZ). In contrast, the heat-affected zone (HAZ) only experiences heating depending on the distance from the fusion line.[7] During non-equilibrium cooling of the welding, the PMZ solidifies through eutectic transformation, creating a ledeburite structure composed of cementite and austenite, where the austenite undergoes eutectoid or martensitic transformation. The ledeburite structure is considered the most brittle structure in cast iron and is highly susceptible to cracking under induced residual stresses of the welding process.[8,9] The most prominent microstructural transformation in the HAZ of DCI is hard un-tempered martensite.[10] Preheating and post-weld heat treatment (PWHT) are commonly used for welding DCI to reduce induced thermal and residual stresses as well as to decrease the brittleness of the developed microstructures in the weld.[11,12,13] However, preheating and PWHT are highly uneconomical and hardly practically feasible for large-scale cast iron structures such as those used in wind turbines.
When cast iron or mild/low carbon steel filler metal (FM) is used for welding cast iron, hard and brittle phases can form in the fusion zone (FZ), leading to cracking and poor mechanical properties.[14,15] Thus, using dissimilar filler metals with Ni content is recommended for welding cast irons to avoid the formation of brittle micro-constituents in the weld.[16] The microstructural evolution of ferritic-pearlitic DCI welds using different welding consumables has been studied in the literature.[14,15,17,18,19,20,21]
El-Banna et al.[14] conducted metallographic observations of single-pass bead-on-plate welds of 5 different filler electrodes, including pure Ni, Ni-Fe alloy, Ni-Cu alloy, stainless steel alloy, and ferritic low carbon steel onto pearlitic DCI using different heat input levels. They reported that the pure Ni and Ni-Fe filler metals could overcome the fusion zone (FZ) problems due to the carbon pick-up from the pearlitic DCI. Regarding the PMZ and HAZ, they compared the welds’ volume fraction of the developed structures (i.e., ledeburite, martensite, and pearlite structures). They reported that the PMZ of the weld deposited by pure Ni weld contains 95 pct ledeburite, while the Ni-Fe filler metal results in 65 pct ledeburite.[14] It is worth noting that the direct comparison of the microstructural evolution of the PMZ and HAZ in El-Banna et al.[14] is not feasible due to the use of different heat input levels to prepare the welds. Pascual et al.[15] reported that using pure Ni filler metal during the welding of DCI prevents the formation of brittle cementite at the HAZ/FZ interface. Furthermore, Suzuki et al.[17] demonstrated that the width of the ledeburite layer in the PMZ decreases with increasing Ni equivalent in the filler wires. However, they did not provide a comprehensive discussion regarding the underlying reasons behind the influence of Ni on ledeburite formation.[15,17]
Increasing the Si content of DCI from approximately 3.2 to 4.3 wt pct leads to an as-cast fully ferritic matrix, which provides high strength levels with significantly improved elongations compared to conventional ferritic-pearlitic DCI.[22,23] In 2012, three grades of DCI with a single-ferrite phase matrix based on the Si solid-solution-strengthened mechanism were designated into the European standard DIN EN 1563:2012[24]; these grades are EN-GJS-450-18, EN-GJS-500-14, EN-GJS-600-10. The machinability of these ferritic grades is significantly enhanced compared to that of the conventional DCI, resulting in cost savings of approximately 10 pct in mechanical machining.[25,26] This improvement is attributed to eliminating the hardness scattering of the ferritic-pearlitic matrix in the fully ferritic matrix, resulting in a more homogeneous hardness distribution.[25]
The high Si content of the solution-strengthened ferritic DCI (SSFDCI) gives rise to unique metallurgical characteristics, leading to notable differences in welding metallurgy compared to the conventional ferritic-pearlitic DCI. Umetani et al.[27] used the synthetic weld thermal cycle method to simulate the microstructural evolution of the HAZ of two grades of DCI with a fully ferritic matrix (EN-GJS-500-14) and a ferritic-pearlitic matrix (EN-GJS-500-7). They reported that the austenitization rate of the SSFDCI grade is lower than that of the conventional DCI, and less martensite structure appears in the simulated HAZ of the SSFDCI.[27]
The austenitization process requires carbon diffusion to the matrix. In the single-phase ferrite matrix, the austenitization rate is limited by the diffusion of carbon from graphite to the matrix.[27] Indeed, the ferrite phase is sluggishly transformed to austenite compared to the pearlitic structure; pearlite features lamellar structures in which the diffusion distance for carbon from cementite to the ferrite is shorter.[16] Furthermore, the higher the Si content of DCI, the higher the eutectoid transformation temperature.[27] Therefore, it would be expected that the temperature range of the HAZ of the SSFDCI will become smaller than that of the conventional ferritic-pearlitic DCI. Suzuki et al.[17] studied the weld structure of two grades of DCI with different Si contents, FCD 450 with 2.54 wt pct Si, and HiSiFCD with 4.97 wt pct Si. According to their findings, the HAZ width in the higher Si content grade is smaller than that of the one with lower Si content.[17] Moreover, the high Si content of DCI widens the temperature range between the stable and metastable eutectic points, thereby reducing the tendency of metastable eutectic solidification and the formation of brittle cementite.[22] Thus, it is reasonable to expect that welding SSFDCI reduces the risk of brittle ledeburite within the PMZ.
Despite the growing applications of the SSFDCI grades in various industries, such as automotive, maritime, and wind turbines,[25,28] only a few studies have been conducted on their welding metallurgy and weldability.[27,29] Therefore, this work investigates the welding metallurgy of SSFDCI by depositing two different filler metals on thick EN-GJS-500-14 plates, using the same heat input level without preheating and PWHT. This study is an in-depth investigation of the metallurgical aspects behind the microstructural evolutions of different regions of the welds, which is crucial for understanding the impact of FMs on weld quality and selecting the most suitable FM for repair and joint welding applications where preheating and PWHT are not feasible.
2 Materials and Experiments
2.1 Materials
Ductile cast iron EN-GJS-500-14 plates with a thickness of 38 mm and plate dimensions of 200 × 85 mm were selected as the base metal (BM). The material complies with DIN EN 1563:2012,[24] and the plates were obtained from a larger cast-on sample (Type D) attached to a bigger component cast in a sand mold. The cast-on samples are commonly used to verify the mechanical and microstructural properties of parts.[24]
A metal-cored wire and a solid wire with the same diameter (1.2 mm) but different chemical compositions were used as FMs. The metal-cored wire was a NiFe alloy containing approximately 53 wt pct iron and 45 wt pct nickel, called “half Ni FM” in this paper. The solid wire was AWS 5.15 ERNi-CI, composed of essentially pure Ni (99 wt pct) with no oxidizers, called “pure Ni FM” in the present paper. Table I shows the base metal’s chemical composition determined using optical emission spectroscopy (OES) and the typical composition of the half Ni FM. The combustion LECO analysis technique was utilized to measure the carbon content precisely.
2.2 Welding Process
Two single-track bead-on plate welds with a length of 15 cm were separately deposited on EN-GJS-500-14 plates using automated metal-cored arc welding (MCAW) and automated gas metal arc welding (GMAW) processes[30,31] by the half Ni and pure Ni FMs, respectively. In the present paper, the weld track deposited by the MCAW process and half Ni FM is called “half Ni weld,” and the track deposited by the GMAW process and pure Ni FM is called “pure Ni weld.” The schematic of the welding process is illustrated in Figure 1(a).
(a) A general circuit schematic of employed welding processes together with the enlarged welding area (reprinted with permission from Ref. [7] the chapter titled “Fusion Welding Processes”), and (b) schematic illustration of the geometrical attributes of a single bead-on-plate weld track on a base metal (BM); H: height, W: width, D: depth, M: substrate-melted zone area, B: build-up zone area, M + B: FZ area, and the BM dilution ratio is calculated by \(\text{pct} D=(\frac{M}{M+B})\times 100\)
The process parameters of both welding processes are summarized in Table II. Two different wire feeding rates were selected to obtain the same welding heat input in both methods due to the different FMs. Since the welding processes were conducted through the synergic feature of a Fronius TransPuls Synergic 4000 welding machine, the welding current and voltage are automatically set by the wire feeding rate. Moreover, the mixture of the shielding gases is different because of the different nature of the filler wires. The other parameters were kept constant in both methods to facilitate the comparison of the metallurgical behavior in welds performed by two filler wires with different chemical compositions.
2.3 Characterization
Standard metallographic procedures were performed on the transverse cross section of both welds, and Nital 2 pct etchant was used to reveal the macro- and microstructure. The geometrical features of the welds, such as height (H), width (W), depth (D), BM dilution ratio (pctD), and fusion zone (FZ) area (see the schematic illustration in Figure 1(b)), were measured on the metallographic cross-section macrostructure using image processing software FIJI ImageJ. Optical microscopy (OM) was used for characterization at low magnification of the developed microstructures in different regions. Scanning electron microscopy (SEM) equipped with energy-dispersive spectroscopy (EDS) was used for a more in-depth study of microstructural evolution.
Microhardness Vickers testing was performed with a holding period of 12 s and different indenter loads depending on the investigated phases or structures. Hardness mappings were conducted using a matrix format of 11 rows and 6 columns, maintaining a 200-micron space between indentations, and a total of 66 indentations were made using a 300gf load. The phase constituent of the fusion zones of both welds was determined by X-ray diffraction using Cu-Kα radiation, and the diffraction angle (2θ) range was 20 to 90 deg.
Thermo-Calc™ software was used to simulate the solidification paths and products of both welds under non-equilibrium Scheil solidification conditions (i.e., no diffusion in the solid state and complete mixing in the liquid). The simulations focused on stable (Fe-Graphite) and metastable (Fe-cementite) systems. Moreover, thermodynamic Scheil simulations were also performed to study the impact of different Ni dilution levels on BM's liquidus temperature and eutectic transformation point. The TCFE12 thermodynamic database was utilized for these simulations. The chemical composition of the FZs was calculated based on the geometrical BM dilution ratio of each weld. It has been confirmed that there is good agreement between the geometrical and chemical composition methods for determining the dilution ratio.[7,32,33] Therefore, the chemical composition of the FZ can be calculated based on the geometry of the weld transverse cross section.
The cross section of both welds was investigated by X-ray tomography using a Zeiss Xradia 520 Versa. The X-ray energy source utilized a polychromatic beam with a maximum energy of 160 kV generated from a tungsten target. A total of 3601 projections were acquired during a full sample rotation of 360 deg, captured by a 2 k × 2 k CCD detector. Reconstruction of 3D maps was accomplished using the Feldkamp, Davis, and Kress algorithm for cone-beam reconstruction,[34] resulting in 2 k × 2 k × 2 k pixel volumes. The volumes were further processed using Avizo 3D software for materials science.
3 Results and Discussions
3.1 Macrostructure of Welds
Figure 2(a) and (b) shows the cross-sectional macrostructure of both single-track welds. The FZ undergoes complete melting and solidification, resulting in a new composition combining the FM and the BM. Adjacent to the fusion boundary, the PMZ is observed as a glossy region where partial melting occurs. The HAZ experiences elevated temperatures based on the proximity to the fusion line but does not melt and, therefore, only undergoes solid-state transformations.
Cross-sectional macrostructure of the weld deposited by (a) half Ni wire and MCAW process (half Ni weld), and (b) pure Ni wire and GMAW process (pure Ni weld). The PMZ is observed as a glossy region along the fusion boundary, and the HAZ is located beneath the PMZ. The solid yellow and white boxes represent where OM and SEM observations were conducted. Furthermore, the white boxes represent the locations where the hardness maps were performed. The yellow arrows in (a) indicate the peninsular macrosegregation
It can be seen in Figure 2(b) that the transverse cross section of the pure Ni weld features a papillary-type penetration pattern in the middle of the weld at the deepest place, which is known as an “Argon finger” (dashed box in Figure 2(b)). Indeed, the high argon concentration in the shielding gas of the GMAW process (see Table II) for depositing the pure Ni FM causes this Argon finger melting profile.[7,35,36,37] It can be seen that the half Ni weld (Figure 2(a)) exhibits a larger FZ area compared to the pure Ni weld (Figure 2(b)), even though both were deposited with the same heat input level. The geometrical attributes of the welds are listed in Table III.
The FZ of the half Ni weld is higher, wider, and larger, while the pure Ni weld is deeper and more diluted with BM due to the “Argon finger” phenomenon. These discrepancies can be related to the different CO2 levels in the shielding gas of the welding processes. Higher CO2 content generates more heat in the melt pool and produces higher melt pool temperatures.[38] Increasing the CO2 content will increase heat transfer to the workpiece due to hotter arc plasma, higher thermal conductivity than argon, and the reaction between CO2 and the melt pool. This leads to more thermal energy transfer from the arc to the melt pool, which results in a larger FZ.[39]
The higher transferred energy to the workpiece with the higher CO2 content results in a wider HAZ in the half Ni weld (Figure 2(a)) compared to the pure Ni weld (Figure 2(b)). The hardness maps across the BM to the FZ of both welds are shown in Figure. 3. These maps confirm that the high hardness region between BM and FZ is wider for half Ni weld compared to the other weld. These hard regions indicate the presence of both PMZ and HAZ, which are not distinguishable based on the hardness map. The microstructural evolutions of these zones are discussed in detail in the following section.
Microhardness map of both welds across the BM to the FZ, where the white boxes are indicated in Fig. 2
3.2 Microstructure of FZs
Figure 4 shows an optical micrograph of the FZ microstructures of the indicated yellow boxes inside the FZs of Figure 2. Both welds exhibit a dendritic-like microstructure comprising an austenitic dendrite core and different interdendritic products. The superimposed SEM micrographs reveal that the interdendritic regions solidify through a eutectic transformation. In the FZ of the half Ni weld (Figure 4(a)), the interdendritic areas are primarily occupied by cementite precipitation. This observation is consistent with the study by El-Banna et al.[11] who reported the formation of ledeburite carbide structures in the FZ of the ferritic-pearlitic DCI using a NiFe FM when no preheating is employed. In contrast, graphite is dominantly formed along the interdendritic regions of pure Ni weld (Figure 4(b)). Furthermore, alongside the interdendritic graphite, dispersed graphite nodules can be observed in the microstructure of the pure Ni weld (Figure 4(b)). The formation of dispersed graphite nodules in the FZ of DCI is also evident in the previous works,[15,21] where a high Ni content electrode was used for the welding of ferritic-pearlitic DCI[15] and ferritic DCI.[21]
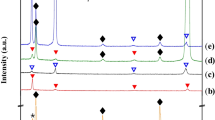
X-ray diffraction patterns of the FZs (Figure 5) indicate the reflection of the austenite (γ) phase in both welds. The FZ of the half Ni weld (Figure 5(a)) also reflects cementite precipitation, while the XRD pattern of the pure Ni weld (Figure 5(b)) is only indexed by the γ phase without any graphite reflection. The absence of graphite peak, normally reflected at 2θ ≈ 26.7, can be attributed to its light density and low volume fraction in the FZ, around 5.8 vol. pct in Figure 4(b).
Based on the hardness maps shown in Figure 3, it can be observed that the half Ni weld has a higher FZ hardness compared to the pure Ni weld. The average FZ hardness of the half Ni weld is 364 ± 17 HV0.3, whereas the pure Ni weld exhibits a softer FZ with a hardness of 181 ± 9 HV0.3. Indeed, the graphite precipitation in the pure Ni weld leads to the softer FZ by inhibiting the formation of hard constituents in the FZ (the microhardness of the interdendritic cementite of the half Ni weld is 710 ± 18 HV0.01). In addition to the softer FZ of the pure Ni weld, graphite precipitation in the FZ increases its volume and reduces melt pool shrinkage. This helps to decrease the thermal and residual stress, eventually lowering the susceptibility to cracking in the cast iron weld.[16]
Figure 6 illustrates the thermodynamic simulation of solidification paths of the FZs in stable (Fe-Graphite) and metastable (Fe-cementite) systems. Accordingly, austenite is the primary solidification phase (L → γ) in both welds; solidification proceeds by following two different systems, resulting in two different interdendritic products. Graphite formation is promoted in the stable system, while cementite precipitation occurs in the metastable system. Based on the microstructural observation (Figure 4), the half Ni weld solidified through the metastable system. In contrast, the pure Ni weld preferred to follow the stable system promoting the formation of graphite. The discrepancy between interdendritic products and solidification paths in the FZ of both welds can be discussed as follows:
During the solidification process, competition arises between stable and metastable systems. In general, the freezing liquid adopts the eutectic structure, requiring lower undercooling.[40] A crucial controlling parameter is the difference between the equilibrium eutectic temperatures of graphite and cementite (ΔTe), with an increased ΔTe leading to a stronger graphitizing effect.[40] Thermodynamic Scheil simulations (Figure 6) indicate that the pure Ni weld exhibits a ΔTe 100 °C higher than the half Ni weld, likely due to variations in the Ni content within the fusion zones (FZs).
The FZ of the pure Ni weld contains approximately 50 wt pct Ni which is twice as much as half Ni weld (25.8 wt pct Ni). Since the γ-Fe and Ni can form a completely miscible solid solution, the austenitic dendrites become further enriched with Ni when pure Ni FM is used. It is well known that alloying elements soluble in austenite raise the graphite eutectic temperature.[40] Moreover, referring to the binary phase diagram of C-Ni and C-Fe systems in [41] γ–Ni has a lower maximum capacity to dissolve C compared to γ–Fe (0.6 pct wt. for the C-Ni system versus 2.1 pct wt. for the C-Fe system). Consequently, the Ni-rich FZ (in the pure Ni weld) exhibits a stronger driving force to reject C toward liquid bulk and interdendritic areas. It is documented that the alloying elements which increase the carbon activity in the liquid bulk contribute to an increased ΔTe.[40] As illustrated in Figure 6, the onset of graphite formation in the pure Ni weld (Figure 6(b)) occurs at fs ≈ 0.036, which is significantly lower than that of the half Ni weld (fs ≈ 0.43 in Figure 6(a)). This indicates that the austenite dendrites in the FZ of the pure Ni weld have a limited solubility for carbon and promptly reject it during the early stages of solidification. Consequently, the FZ of the pure Ni weld exhibits a strong tendency to solidify through the stable (Fe-Graphite) system, resulting in graphite precipitation at high temperatures.
The formation of the eutectic cementite in the metastable (Fe-cementite) system requires significant undercooling,[2,42] which can be achieved during low heat input welding due to rapid solidification. As mentioned above the ΔTe of half Ni weld is much lower than that of the pure Ni weld. Moreover, it is well known that during the solidification of cast iron cementite is predominately formed at high cooling rates while graphite is obtained at low cooling rates.[43] The dendrite arm spacing decreases with an increasing cooling rate.[7] It is evident in Figure 4 that the dendritic cells of the half Ni weld are much finer than that of the pure Ni weld, indicating that the half Ni weld solidified through higher cooling rates. Furthermore, the temperature variations of L → γ during the solidification around the fs of the graphite formation are more pronounced in Figure 6(a) (half Ni weld) compared to Figure 6(b) (pure Ni weld), facilitating the required undercooling for cementite formation. Therefore, the FZ of the half Ni weld prefers to follow the metastable (Fe-cementite) system to precipitate cementite rather than graphite.
3.3 HAZ Microstructure of Half Ni Weld
Figure 7 shows the BM microstructure of the investigated EN-GJS-500-14 substrate, indicating an as-cast fully ferritic matrix coupled with spheroidal graphite nodules. The hardness of the BM is 183 ± 4 HV3.0 while the hardness of the fully ferritic matrix is 250 ± 12 HV0.3 when avoiding the graphite nodules.
The BM microstructure changes in the HAZ depending on the distance to the fusion line. Figure 8(a) shows the microstructure of the BM, HAZ, PMZ, and FZ in the half Ni Weld (indicated with a white box in Figure 2(a)). Far from the fusion zone, upon heating the transformation of Ferrite → Austenite commences in the grain boundaries as well as around the graphite nodules. The transformation is then reversed upon cooling by forming a martensite structure with fine lathes from the previously formed austenite (Figure 8(b)).
In the absence of pearlite in the BM matrix of SSFDCI, the graphite nodules play a crucial role in carbon release. More C diffuses out from the graphite nodules located closer to the fusion line as they experience higher peak temperatures during the heating cycle of the welding process.[44] As shown in Figure 8(c), approaching the fusion line, the larger the area around the graphite nodules is transformed to martensite. Further away from the nodules, the unaffected ferritic matrix can be observed. However, most of the BM matrix transforms right beneath the PMZ in Figure 8(a) since the peak temperatures and the duration above the critical temperature A1 are sufficient to induce complete transformation into austenite.
Figure 8(d) presents the microstructure of the matrix indicating martensite structure development with fine lathes, whereas the HAZ martensite structure becomes coarser in the region closer to the PMZ (see Figure 8(e)). The α΄martensite structure morphology changes from fine lath type (Figure 8(d)) to lenticular type (Figure 8(e)) by getting closer to the fusion line.[45] Indeed, the closer the region is to the fusion line, the more carbon is released into the matrix. Consequently, the formation of the coarser martensite is promoted due to the higher C content coupled with the lower martensite transformation starting temperatures (Ms point).[44,45] In comparison with other ferritic-pearlitic DCI, assuming the same welding condition, the HAZ of the SSFDCI is narrower than that of the ferritic-pearlitic DCI.[11]
3.4 PMZ Microstructure of Half Ni Weld
At the PMZ, the experienced temperatures are high enough to melt some parts of the material adjacent to the fusion line. At the same time, the remaining unmelted portions transform to austenite at elevated temperatures. The unmelted parts are rich in carbon, and therefore, as discussed above, the coarse lenticular martensite structure develops close to the fusion line (see Figure 8(f)). Moreover, proeutectoid cementite can be observed along the grain boundaries of Figure 8(f). This microstructure is attributed to the amount of C content freed into the matrix being beyond the maximum solubility in γ–Fe, and the excess C might not be able to diffuse back to the pre-existing graphite nodules due to the high cooling rates. Consequently, the free C atoms accumulate along the grain boundaries through the diffusion and grain growth sweeping mechanisms, leading to the precipitation of proeutectoid cementite.
The melted portions of the PMZ solidify through eutectic transformation, forming a ledeburite structure comprising cementite and austenite. Subsequently, the austenite undergoes either eutectoid or martensitic transformation depending on the chemical composition and the cooling rates. Figure 8(g) shows a high-magnification optical micrograph of the PMZ indicating the ledeburite structure, consisting of mostly elongated cementite and some martensitic areas. Figures 8(h) and (i) are images with higher magnification of the ledeburite structure, confirming its lamellar eutectic pattern of cementite and martensite. Moreover, a pearlite structure can be observed between some of the cementite layers of Figure 8(i). However, the martensite structure is the governing transformation of the eutectic austenite phase of the ledeburite structure due to the high cooling rates of the investigated welds, which were conducted using low heat input level at room temperature (no preheat condition).
Figure 8(j) presents an SEM micrograph of a graphite nodule in the PMZ showing the martensite structure formed around the graphite nodule and then surrounded by the ledeburite structure. Moreover, according to Figure 8(a), most graphite nodules inside the HAZ and PMZ are mainly surrounded by the martensite structure. The absence of ferrite or pearlite layers around most of the graphite nodules in the HAZ and PMZ implies that the cooling rate in these zones was relatively rapid, preventing sufficient diffusion of carbon atoms back toward the graphite nodules during the cooling cycle. Consequently, forming a ferrite halo around the graphite nodules was hindered.
3.5 HAZ & PMZ Microstructure of Pure Ni Weld
Figure 9(a) shows the microstructure of the BM, HAZ, PMZ, and FZ in the pure Ni weld (indicated with a white box in Figure 2(b)). The microstructural evolution of the HAZ and PMZ, including the formation of martensite and ledeburite structures, exhibits similarities to those observed in the half Ni weld. The following section focuses on the hardness measurements of the phases and structures within the HAZ and PMZ of the pure Ni weld, which is more or less similar to the half Ni weld.
(a) Optical microstructure profile of pure Ni weld across the BM to the FZ, (b), (c), (d), (e), (f), and (g) are microhardness indentations of indicated boxes in (a), (h) and (i) are EDS analysis results of the boxes designated boxes in (f) and (g), respectively. The EDS results are provided for comparative purposes only
In Figure 9(b), a hardness indentation on the ferritic matrix of the HAZ yields a hardness value of 243 HV0.3. This hardness level is in the range of the ferritic matrix of BM (250 ± 12 HV0.3), indicating that the unaffected matrix within the HAZ does not contribute significantly to the hardening mechanism of the HAZ.
The hardness of the martensite island in Figure 9(c) is 330 HV0.3, while closer to the fusion line, the hardness of martensite increases to 575 HV0.3 (Figure 9(d)). Figure 9(e) shows that the hardness of lenticular martensite corresponds to 826 HV0.3. The variations of hardness across the HAZ support the explained microstructural evolution, where the closer the distance to the fusion line, the larger the carbon release and, therefore, the harder the developed martensite.
The indentation on the ledeburite structure in the PMZ (Figure 9(f)) results in 1003 HV0.3, corresponding to what should be expected in white cast iron. However, closer to the FZ (Figure 9(g)), the hardness drops to 454 HV0.3. This region retains the lamellar eutectic structure of the PMZ but is coarser and more like a dendritic structure. Interestingly, the austenite phase remains between the eutectic layers instead of transforming into the martensite or pearlite structures (as observed in Figures 8(h) and (i)).
Figures 9(h) and (i) present the EDS analysis of the designated boxes next to the indentation points in Figures 9(f) and (g), respectively. The EDS results reveal a significantly higher concentration of Ni element next to the hardness point of 454 HV0.3 (19.3 pct wt. Ni, Figure 9(g)) compared to the point of 1003 HV0.3 (2 pct wt. Ni. Figure 9(f)). Since Ni is an austenite stabilizing element and can form a fully miscible solid solution with γ-Fe, the eutectic austenite phase remains austenitic during cooling in the lamellar structure in the region with higher Ni content, leading to the reduction of hardness (Figures 9(g) and (i)).
Figures 10(a) and (b) illustrate EDS line scan analyses of Fe, Ni, and C from the HAZ toward the FZ of the half Ni and pure Ni welds, respectively. The elemental distribution trends exhibit relatively uniform patterns in the FZs. It is worth noting that encountering two small graphite nodules within the FZ of the pure Ni weld along the EDS line path leads to two peaks in the C distribution trend, which also causes variation in the Ni and Fe trends. The FZ trends confirm that the FZ of the pure Ni weld contains a higher concentration of Ni than that of the half Ni weld.
According to Figures 10(a) and (b), the FZ composition trends are followed by a sharp transition zone (TZ) reaching the PMZ. This transition happens due to the partial mixing of the molten material of the FZ and the melted BM layer along the fusion boundary and elemental diffusion between them.[46] Following the sharp TZ is the PMZ, where the Ni concentration gradually decreases, reaching zero, at a shorter distance for half Ni weld while a longer length for the pure Ni weld. The higher concentration of Ni in the pure Ni weld results in a stronger driving force for Ni diffusion into the PMZ, leading to a longer diffusion distance.
Figure 11 illustrates the results of the thermodynamic Scheil simulation, showing the variations of the TL and fs for eutectic cementite formation (fs-cem) in EN-GJS-500-14 BM with different Ni dilution ratios. It is evident that an increase in Ni content leads to higher TL and fs-cem. This implies that the diluted PMZ by Ni experiences higher TL and the formation of the ledeburite structure (eutectic cementite, fs-cem) is delayed upon cooling.
The higher TL can potentially result in melting the unmelted portion of the PMZ. This explains the difference in structure observed in region g of Figure 9 (with 17.4 pct wt. Ni) where there are no unmelted martensite islands compared to the PMZ in region f of Figure 9 (with 1.8 pct wt. Ni). Indeed, the higher TL in these regions caused by the presence of Ni facilitates the complete melting of the PMZ. Furthermore, Ni stabilizes the austenite phase, delaying the formation of eutectic cementite (higher fs-cem), resulting in the solidification of more austenite and less cementite along the fusion boundary.
Therefore, the higher TL coupled with higher fs-cem due to the diffusion of Ni into the PMZ for pure Ni weld avoids developing the brittle ledeburite structure along the fusion boundary, leading to a more ductile interface with the FZ. Comparison of Figure 2(a) with Figure 2(b) as well as Figure 8(a) with Figure 9(a), reveals that the PMZ is not developed as extensively along the fusion boundary in the pure Ni weld (Figures 2(b) and 9(a)) compared to the half Ni weld (Figures 2(a) and 8(a)).
Thus, adequate penetration of Ni into the PMZ can effectively inhibit the formation of brittle structures along the fusion boundary, which can be achieved using an FM with a high Ni content. This observation aligns with the previous works,[15,17] reporting that using high Ni FM restricts the formation of the cementite layer in the PMZ, although their analyses did not scrutinize the root cause for this outcome.
3.6 Macrosegregation
According to the macrograph of the half Ni weld (Figure 2(a)), two portions of the PMZ are observed to be drawn into the FZ (indicated by arrows inside the FZ) in the form of a peninsula. Generally, in dissimilar welding solute segregation can occur in the macroscopic scale to form macrosegregation patterns in the FZ; this phenomenon has also been called unmixed zone (UZ), filler-metal-depleted area, partially mixed zone, intermediate mixed zone, and hard zone.[7] Figure 12(a) illustrates a peninsular macrosegregation of the half Ni weld, which has been dragged from the fusion boundary into the FZ. Indeed, the weld pool convection has the potential to carry a portion of the PMZ into the FZ, where it rapidly solidifies to form a peninsula before sufficient mixing can occur.
The microhardness measurements along the peninsula (see Figure 12(a)) confirm that it is a hard structure incorporated into the softer FZ. Moreover, the EDS line scan analysis (Figure 12(b)) crossing the peninsula inside the FZ exhibits a distinct chemical composition compared to the FZ. The peninsula demonstrates lower Ni concentration and higher Fe content than the surrounding FZ.
It is expected that the beach-type UZ must also form along the fusion boundary. However, differentiating it from the PMZ in the cross section is challenging since both experience melting and re-solidification with a composition similar to BM. The peninsula-type macrosegregation is more distinguishable due to its distinctive pattern. X-ray computed tomography offers improved visualization for observing the development of macrosegregation along the fusion boundary and within the FZ of the half Ni weld at various cross-sections (Figures 13(a) through (c) and the supplementary video 1).
X-ray tomography images in different cross-sections of the half Ni weld: (a) a cross section illustrating the UZ in the form of a beach and peninsula, (b) a cross section located at a distance of 425 µm from (a), (c) a cross section located at a distance of 1225 µm from (a), and (d) an X-ray tomography image of a cross section of the pure Ni weld. The corresponding videos are available in the supplementary file, supplementary Video 1 for (a–c) and supplementary video 2 for 13(d)
X-ray tomography acquires images by detecting the transmission of X-rays through the specimen. The absorption of X-rays is influenced by the density and thickness of the material, resulting in variations in image intensity. Lighter materials absorb fewer X-rays, appearing as darker regions in the images, while denser materials exhibit higher X-rays absorption and appear brighter. In the case of the investigated specimen, the graphite nodules, having lower density than the ferritic matrix, appear as darker phases in the X-ray images. The solidified materials along the fusion boundary (PMZ and beach-type UZ) are also observed as a darker region because the composition is mostly similar to BM. Furthermore, the UZ peninsula, which carries liquified material along the fusion boundary into the FZ, is also visible as a darker region within the FZ.
Figure 13(d) presents an XCT image of a cross section of the pure Ni weld, showing no significant macrosegregation in the form of peninsulas (see supplementary video 2). Indeed, the formation of the UZ in the form of peninsulas was exclusively observed in the half Ni weld. The formation of the UZ during dissimilar welding can be explained based on the differences in liquidus temperatures between the FZ (TLFZ) and the BM (TLBM), as documented in the literature.[46,47,48]
The peninsular macrosegregation comes up from the fusion boundary to the FZ with structure and hardness characteristics similar to the PMZ. Therefore, the formation of the UZ peninsula is directly related to the presence of PMZ itself. In the half Ni weld, where there is a lower diffusion of Ni into the PMZ, see Figure 10(a), the formation of the PMZ cannot be avoided. As a result, during solidification, the weld pool fluid flow carries a portion of the PMZ from the fusion boundary into the FZ, forming the UZ peninsula due to insufficient mixing time. On the other hand, in the pure Ni weld, Ni diffuses more into the PMZ (see Figure 10(b)), inhibiting the formation of the brittle PMZ due to the higher TL and fs-cem. As a result, the UZ peninsula is not observed in the pure Ni weld.
4 Conclusions
In summary, this study extensively investigated the microstructural evolution during the single-track bead-on-plate welding of high Si solution-strengthened ferritic DCI using two filler metals with different Ni contents (half Ni and pure Ni) under the same welding heat input. The primary conclusions emerging from this research are as follows:
-
1.
The FZ of both welds exhibited a dendritic-like microstructure comprising an austenitic dendrite core and different interdendritic products. The interdendritic areas of the half Ni weld were primarily occupied by precipitation of eutectic cementite (average FZ hardness of 364 ± 17 HV0.3), while the FZ of the pure Ni weld featured interdendritic graphite, accompanied by dispersed graphite nodules (average hardness of 181 ± 9 HV0.3). Indeed, the FZ of the half Ni weld solidified through the metastable (Fe-cementite) system, whereas the FZ of the pure Ni weld solidified within the stable (Fe-Graphite) system, resulting in high-temperature graphite precipitation. The thermodynamic Scheil simulation revealed that this discrepancy is due to the Ni-enriched FZ of the pure Ni weld having a larger ΔTe and limited solubility for C and promptly rejecting it during the early stages of solidification.
-
2.
The formation of martensite structure was the predominant microstructural evolution in the HAZ of the EN-GJS-500-14 BM. The martensite transformation initiated around the graphite nodules and grain boundaries, gradually spreading to a larger area as it approached the fusion line. Ultimately, beneath the PMZ, most of the BM matrix underwent a martensitic transformation. The martensite structure morphology changed from fine lath type to lenticular type as it got closer to the fusion line, accompanied by increased martensite hardness.
-
3.
The unmelted part of PMZ featured a coarse lenticular martensite structure coupled with proeutectoid cementite along the grain boundaries. While the melted part solidified as a lamellar ledeburite structure comprising cementite and primarily martensite. The diffusion of Ni from the FZ into the PMZ was driven by the Ni content of the FZ so the diffusion occurred at a shorter distance for half Ni weld while a longer length for the pure Ni weld. The presence of more Ni in the PMZ of the pure Ni weld prevents the widening of the layer of the brittle structures along the fusion boundary, leading to a softer interface with the FZ in the pure Ni weld.
-
4.
The thermodynamic Scheil simulation revealed that an increase in the Ni content resulted in a higher liquidus temperature (TL) and a higher mole fraction of solid for eutectic cementite formation (fs-cem). Higher TL facilitates the complete melting of the PMZ, and the higher fs-cem delays the formation of the eutectic cementite. Therefore, the higher TL coupled with higher fs-cem due to the diffusion of Ni into the PMZ of pure Ni weld effectively prevented the formation of brittle PMZ along the fusion boundary.
-
5.
The unmixed zone in the form of peninsular macrosegregation observed in the half Ni weld, which is not documented in the existing literature focusing on the welding of DCI. Indeed, in the half Ni weld, where the diffusion of Ni into the PMZ is insufficient, the formation of PMZ cannot be avoided. As a result, during solidification, the weld pool fluid flow carries a portion of the brittle PMZ into the FZ, forming peninsular macrosegregation due to limited mixing time. In contrast, such macrosegregation was not observed in the pure Ni weld.
-
6.
To compare the microstructural evolution between SSFDCI and typical DCI, assuming identical welding conditions, it can be inferred that the FZ may solidify through a similar phase transformation sequence. However, the HAZ in SSFDCI is narrower compared to the pearlitic and ferritic-pearlitic grades of DCI. This is attributed to the fully ferritic matrix of SSFDCI, which undergoes a slower transformation into austenite.
In conclusion, the microstructural observation revealed that the Ni-enriched FM offers significant advantages compared to the NiFe FM. It results in a softer FZ without brittle constituents along the interdendritic regions, a narrower HAZ and PMZ, and eliminates the issue of macrosegregation of brittle fusion boundary products into the FZ. As a result, the Ni-enriched FM emerges as the superior choice for direct deposition as a butter layer in repair or joint welding applications involving the EN-GJS-500-14 base metal.
Data Availability
Data will be made available on request.
References
Y.B. Zhang, T. Andriollo, S. Fæster, R. Barabash, R. Xu, N. Tiedje, J. Thorborg, J. Hattel, D. Juul Jensen, and N. Hansen: Acta Mater., 2019, vol. 167, pp. 221–30.
N.S. Tiedje: Mater. Sci. Technol., 2010, vol. 26, pp. 505–14.
M. Shirani and G. Härkegård: Eng. Fail. Anal., 2010, https://doi.org/10.1016/j.engfailanal.2010.07.001.
K.M. Pedersen and N.S. Tiedje: Mater Charact, 2008, vol. 59, pp. 1111–21.
C.M. Lin, A.S. Chandra, L. Morales-Rivas, S.Y. Huang, H.C. Wu, Y.E. Wu, and H.L. Tsai: Int. J. Cast Met. Res., 2014, vol. 27, pp. 378–83.
F. Malek Ghaini, M. Ebrahimnia, and S. Gholizade: Eng. Fail. Anal., 2011, vol. 18, pp. 47–51.
S. Kou: Welding Metallurgy, 2nd ed. Wiley, New Jersey, 2003.
N. Sommer and S. Böhm: J. Adv. Join. Process., 2022, vol. 5, p. 100078.
Z. Weng, A. Wang, Y. Wang, D. Xiong, and H. Tang: Surf. Coat. Technol., 2016, vol. 286, pp. 64–71.
Y. Li, S. Dong, S. Yan, P. He, and B. Xu: Surf. Coat. Technol., 2018, vol. 339, pp. 37–47.
E.M. El-Banna: Mater. Lett., 1999, vol. 41, pp. 20–26.
R.C. Voigt and C.R. Loper: Weld. J., 1983, vol. 62, p. 82.
E.S.V. Marques, F.J.G. Silva, O.C. Paiva, and A.B. Pereira: Materials, 2019, https://doi.org/10.3390/ma12142263.
E.M. El-Banna, M.S. Nageda, and M.M. Abo El-Saadat: Mater. Lett., 2000, vol. 42, pp. 311–20.
M. Pascual, J. Cembrero, F. Salas, and M.P. Martínez: Mater. Lett., 2008, vol. 62, pp. 1359–62.
M. Pouranvari: Mater. Des., 2010, vol. 31, pp. 3253–58.
T. Suzuki, S. Hiratsuka, H. Horie, S. Moriya, and M. Nakamura: J. Jpn. Found. Eng. Soc., 2005, vol. 77, pp. 314–19.
M. Ebrahimnia, F.M. Ghaini, S. Gholizade, and M. Salari: Mater. Des., 2012, vol. 33, pp. 551–56.
M. Zuk, J. Górka, R. Dojka, and A. Czupryński: in IOP Conference Series: Materials Science and Engineering, vol. 227, Institute of Physics Publishing, 2017.
Z. Lestan, M. Milfelner, J. Balic, M. Brezocnik, and I. Karabegovic: Int. J. Adv. Manuf. Technol., 2013, vol. 66, pp. 2023–28.
M. Askari-Paykani, M. Shayan, and M. Shamanian: J. Iron Steel Res. Int., 2014, pp. 252–63.
D. Franzen, K. Weiß, J. Gundlach, and A. Bührig-Polaczek: Int. J. Metalcast., 2020, vol. 14, pp. 1041–51.
T. Ikeda, T. Umetani, N. Kai, N.A. Noda, and Y. Sano: ISIJ Int., 2016, vol. 56, pp. 868–74.
DIN EN 1563:2012 “Founding–Spheroidal graphite cast irons”, 2012.
D. Franzen, P. Weiß, B. Pustal, and A. Bührig-Polaczek: Int. J. Metalcast., 2020, vol. 14, pp. 1105–14.
W. Menk: Materials Science Forum, vol. 925, Trans Tech Publications Ltd, MSF, 2018, pp. 224–30.
T. Umetani, H. Takada, T. Ikeda, T. Yamaguchi, H. Era, and K. Nishio: Weld. Int., 2015, vol. 29, pp. 342–48.
M. Riebisch, P. Weiß, C. Cremer, I. Aboulkhair, and A. Bührig-Polaczek: Mater. Sci. Technol., 2018, vol. 34, pp. 725–30.
E.B. Farahani, A. Sarhadi, M. Alizadeh-Sh, S. Fæster, H.K. Danielsen, and M.A. Eder: J. Manuf. Process., 2023, vol. 104, pp. 443–59.
N.Q. Trinh, S. Tashiro, T. Suga, T. Kakizaki, K. Yamazaki, A. Lersvanichkool, H. Van Bui, and M. Tanaka: Metals (Basel), 2022, https://doi.org/10.3390/met12101577.
S. Kotsakis and K. Drosos: Chalmers University of Technology, 2014.
É.M. Miná, Y.C. da Silva, J. Dille, and C.C. Silva: Metall. Mater. Trans. A, 2016, vol. 47A, pp. 6138–47.
M. Alizadeh-Sh, S.P.H. Marashi, E. Ranjbarnodeh, R. Shoja-Razavi, and J.P. Oliveira: Opt. Laser Technol., 2020, vol. 128, p. 106244.
L.A. Feldkamp, L.C. Davis, and J.W. Kress: Practical Cone-Beam Algorithm, vol. 1, 1984.
A. Joseph, D. Farson, D. Harwig, and R. Richardson: Sci. Technol. Weld. Join., 2005, vol. 10, pp. 311–18.
R. Zong, J. Chen, and C. Wu: J. Mater. Process. Technol., 2020, https://doi.org/10.1016/j.jmatprotec.2020.116781.
B. Mvola and P. Kah: Int. J. Adv. Manuf. Technol., 2017, vol. 88, p. 88.
B. Silwal, A. Nycz, C.J. Masuo, M.W. Noakes, D. Marsh, and D. Vaughan: Int. J. Adv. Manuf. Technol., 2020, vol. 108, pp. 1285–96.
L.L. Wang, F.G. Lu, H.P. Wang, A.B. Murphy, and X.H. Tang: J. Phys. D Appl. Phys., 2014, https://doi.org/10.1088/0022-3727/47/46/465202.
P. Magnin and W. Kurz: Metall. Trans. A, 1988, vol. 19, pp. 1965–71.
ASM Handbook: in Alloy Phase Diagram, vol. 3, ASM International, 2004.
L. Battezzati, M. Baricco, and S. Curiotto: Acta Mater., 2005, vol. 53, pp. 1849–56.
P. Magnin and W. Kurz: Metall. Trans. A, 1988, vol. 19, pp. 1955–63.
S. Itomura, K. Heshiki, F. Matsuda, and Y. Kikuta: Weld. Int., 1989, vol. 3, pp. 872–77.
T. Maki: in Phase Transformations in Steels, Elsevier, 2012, pp. 34–58.
M. Alizadeh-Sh, S.P.H. Marashi, E. Ranjbarnodeh, R. Shoja-Razavi, and J.P. Oliveira: J. Laser Appl., 2020, vol. 32, p. 022048.
T. Soysal, S. Kou, D. Tat, and T. Pasang: Acta Mater., 2016, vol. 110, pp. 149–60.
S. Kou and Y.K. Yang: Weld. J., 2007, vol. 86, p. 303.
Acknowledgments
The presented results are derived from the WeldCast project, funded by the Innovation Fund Denmark Grant Number 0224-00116A; the support is gratefully acknowledged. The authors would like to thank Rian Holdstock and Kaare Jensen from FORCE Technology for their practical support and preparation of the welds. Furthermore, the authors appreciate the technical advice suggested by Tea Soee-Rabjerg and Santiago Castillo Riano from Siemens Gamesa Renewable Energy A/S.
Funding
Open access funding provided by Technical University of Denmark.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file1 (MP4 9470 kb)
Supplementary file2 (MP4 2521 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Alizadeh-Sh, M., Fæster, S., Farahani, E.B. et al. Microstructural Evolution During Welding of High Si Solution-Strengthened Ferritic Ductile Cast Iron Using Different Filler Metals. Metall Mater Trans A 55, 2309–2323 (2024). https://doi.org/10.1007/s11661-024-07399-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11661-024-07399-4