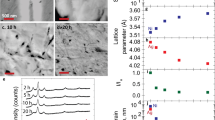
Abstract
To address the challenges associated with the fabrication of highly O2-permeable Ag membranes suitable for on-site and downsized O2 separators for large-scale industrial applications, we investigated nanocrystalline heat-resistant Ag–Cu–Al alloy thin films. Ag–1 at. pct. Al and Ag–2 at. pct. Cu–1 at. pct. Al alloy thin films with nanocrystalline structures were prepared through a reactive sputtering method employing Ar–O2 mixed gas under the condition of supplying excess O/Al flux ratio above the stoichiometric ratio of Al2O3 (> 3/2). To examine the thermal stability of the nanocrystalline Ag alloy films, heating tests were conducted at 350 °C for 1 hour and 300 °C for 1 to 1000 hours in air, and the Ag crystal grain size, nanoindentation hardness, Young’s modulus, and electrical resistivity were measured. The results show that the Ag–2 at. pct. Cu–1 at. pct. Al thin film has excellent thermal stability, maintaining its nanostructure with an average grain size of 30 nm after heating at 300 °C for 1000 hours in air. The effects of the addition of 2 at. pct. Cu and the O2 partial pressures during sputtering on the thermal stability of the Ag nanocrystalline structures were discussed using model calculations. It was found that supplying excess O/Al flux ratio above the stoichiometric ratio of Al2O3 (> 3/2) during sputtering reduced the dissolved Al concentration at the Ag grain boundary to substantially zero, almost completely suppressed the Ostwald ripening of nano-sized Al2O3 particles during sputtering and long-term heating, and allowed the Al2O3 particles to maintain high pinning force. Even under the excess oxygen supply conditions of O/Al > 3/2, the 2 at. pct. Cu addition was preferred for further Ag grain refinement in terms of promoting Al2O3 nucleation at the early stage of the film deposition on the substrate due to the effect of suppressing recrystallization of the Ag matrix.













Similar content being viewed by others
Abbreviations
- E :
-
Young’s modulus (Pa)
- H IT :
-
Nanoindentation hardness (Pa)
- H V :
-
Vickers hardness (kgf/mm2)
- J i :
-
Flux of element i (mol/m2/s)
- P :
-
Sputtering power (W)
- \( {P_{{{\text{O}}_{2} }} } \) :
-
Partial pressure of O2 (Pa)
- R e :
-
Grain boundary reflection coefficient of free electron (–)
- SR :
-
Sputtering deposition rate (m/s)
- T :
-
Temperature (K)
- X i :
-
Mole fraction of element i dissolved in the Ag film (–)
- X i (av.):
-
Average mole fraction of element i dissolved in the Ag grain boundary (–)
- X i (eq.):
-
Equilibrium mole fraction of element i dissolved in the Ag film (–)
- b :
-
Burgers vector (m)
- d :
-
Film thickness (m)
- d g :
-
Grain size of the Ag crystal (m)
- d g (hypo):
-
Hypothetical grain size of the Ag crystal over the entire Ag film (m)
- d p :
-
Particle size of Al2O3 (m)
- f gb :
-
Area fraction of the Ag grain boundary in the cross-section parallel to the Ag film surface (–)
- f p :
-
Area fraction of the Al2O3 particle (–)
- s p :
-
Spacing of Al2O3 particles at the Ag grain boundary (m)
- \({s}_{p}^{0}\) :
-
Initial spacing of Al2O3 particles nucleated at the Ag grain boundary (m)
- s p (hypo):
-
Hypothetical spacing of Al2O3 particles over the entire Ag film (m)
- t :
-
Elapsed time for sputtering or heating time (s)
- δ :
-
Grain boundary width of Ag (m)
- λ e :
-
Mean free path of the free electron (m)
- ρ :
-
Electrical resistivity of the Ag film (Ω·m)
- ρ d :
-
Dislocation density (1/m2)
- ρ matrix :
-
Electrical resistivity of the Ag matrix (Ω·m)
- ρ 0 :
-
Electrical resistivity of Ag annealed bulk (Ω·m)
- ρ p :
-
Electrical resistivity of Al2O3 (Ω·m)
- σ :
-
Tensile strength (Pa)
- \({\varphi }_{{\text{p}}}\) :
-
Volume fraction of the Al2O3 particles in the Ag film (–)
References
National Energy Technology Laboratory, U. S. Department of Energy: Commercial Technologies for Oxygen Production, https://www.netl.doe.gov/research/Coal/energy-systems/gasification/gasifipedia/commercial-oxygen. Accessed 1 Sept 2023
P. Rao and M. Muller: Industrial Oxygen: Its generation and Use, American Council for an Energy-Efficient Economy, 2007, https://www.aceee.org/files/proceedings/2007/data/papers/78_6_080.pdf. Accessed 1 Sept 2023
R. Kiebach, S. Pirou, L.M. Aguilera, A.B. Haugen, A. Kaiser, P.V. Hendriksen, M. Balaguer, J. García-Fayos, J.M. Serra, F. Schulze-Küppers, M. Christie, L. Fischer, W.A. Meulenberg, and S. Baumann: J. Mater. Chem. A, 2022, vol. 10, pp. 2152–95.
M. Jha and N. Gaur: J. Fam. Med. Prim. Care., 2022, vol. 11, pp. 1231–36.
H. Aljaghoub, S. Alasad, A. Alashkar, M. AlMallahi, R. Hasan, K. Obaideen, and A.H. Alami: Int. J. Thermofluid, 2023, vol. 17, 100261.
H. Murakami, M. Fujioka, M. Hase, T. Saito and J. Hayashi: Development of oxygen COG combustion system for steel reheating, the American Flame Research Committee, 1996, https://collections.lib.utah.edu/ark:/87278/s6w37zxz. Accessed 1 Sept 2023
Y. Ueshima, M. Hasegawa, N. Kubota, Y. Matamura, E. Matsubara, K. Seki, and T. Hirato: Materialia, 2023, vol. 30, 101853.
S. Hori, H. Tai, and S. Kawaguchi: J. Soc. Mat. Sci. Jpn., 1981, vol. 30, pp. 556–61.
H. Yavas: Mechanical behavior of nanotwinned materials – experimental and computational approaches, PhD. Thesis, Iowa State University, 2016, pp. 50–60. https://doi.org/10.31274/etd-180810-5670. Accessed 1 Sept 2023
ASTM International: ASTM E112-13, Standard Test Methods for Determining Average Grain Size (2021), https://standards.globalspec.com/. Accessed 1 Sept 2023
W. S. Rasband, ImageJ (U. S. National Institutes of Health, 1997-2012), https://imagej.nih.gov/ij/. Accessed 1 Sept 2023
International Organization for Standardization: ISO 14577-1:2015, Metallic materials — Instrumented indentation test for hardness and materials parameters—Part 1: Test method. https://www.iso.org/standard/56626.html. Accessed 1 Sept 2023
ASTM International: ASTM F374-02, Standard Test Method for Sheet Resistance of Silicon Epitaxial, Diffused, Polysilicon, and Ion-implanted Layers Using an In-Line Four-Point Probe with the Single-Configuration Procedure (2017), https://www.astm.org/f0374-02.html. Accessed 1 Sept 2023
B.K. Kad and P.M. Hazzledine: Mater. Sci. Eng. A, 1997, vol. 238, pp. 70–7.
D. Tabor: The Hardness of Metals, Oxford University Press, Oxford, 1951, pp. 155–71.
T. Sawa: in Proc. of IMEKO2010 TC3,TC5 and TC22 Conferences in Metrology in Modern Context, 2010, pp. 171–74, https://www.imeko.org/publications/tc5-2010/IMEKO-TC5-2010-009.pdf. Accessed 1 Sept 2023
J.W. Aldrich and R.W. Armstrong: Metall. Trans., 1970, vol. 1, pp. 2547–50.
M. F. Ashby: Physics of Strength and Plasticity, ed. By A. S. Argon, MIT Press, Cambridge, MA, 1969, pp. 113–31.
Smithells Metals Reference Book 7th ed., Ed. By E.A.Brandes and G.B.Brook, Butterworth-Heinemann, Oxford, Oxon, 1994, p. 15-3.
Smithells Metals Reference Book 7th ed., Ed. By E.A.Brandes and G.B.Brook, Butterworth-Heinemann, Oxford, Oxon, 1994, p. 4–24.
J.E. Bailey and P.B. Hirsch: Philos. Mag., 1960, vol. 5, pp. 485–97.
H. Zhang, J. Geng, R.T. Ott, M.F. Besser, and M.J. Kramer: Metall. Mater. Trans. A, 2015, vol. 46A, pp. 4078–85.
S. Jalili, F. Hajakbari, and A. Hojabri: J. Theor. Appl. Phys., 2018, vol. 12, pp. 15–22.
D.S. McLachlan, M. Blaszkiewicz, and R.E. Newnham: J. Am. Ceram. Soc., 1990, vol. 73, pp. 2187–03.
A.F. Mayadas and M. Shatzkes: Phys. Rev. B, 1970, vol. 1, pp. 1382–9.
J.W.C. De Vries: Thin Solid Films, 1988, vol. 167, pp. 25–32.
E.I. Tochitskii and N.M. Belyavskii: Phys. Stat. Sol. (A), 1980, vol. 61, pp. K21-4.
N. Artunç, M.D. Bilge, and G. Utlu: Surf. Coat. Technol., 2007, vol. 201, pp. 8377–1.
K. Barmak, A. Darbal, K.J. Ganesh, P.J. Ferreira, J.M. Rickman, T. Sun, B. Yao, A.P. Warren, and K.R. Coffey: J. Vac. Sci. Technol., 2014, vol. 32, p. 061503.
G.S. Jang, D.Y. Kim, and N.-M. Hwang: Electron. Mater. Lett., 2021, vol. 17, pp. 172–80.
L.A. Zepeda-Ruiz, G.H. Gilmer, C.C. Walton, A.V. Hamza, and E. Chason: J. Cryst. Growth, 2010, vol. 312, pp. 1183–7.
K. Mizukoshi, T. Yamamura, Y. Tomioka, and M. Kawamura: Jpn. J. Appl. Phys., 2022, vol. 61, 095503.
N. Sano, S. Honma, and Y. Matsushita: Met. Trans., 1970, vol. 1, pp. 301-.
R.A. Outlaw, S.N. Sankaran, G.B. Hoflund, and M.R. Davidson: J. Mater. Res., 1988, vol. 3, pp. 1378–84.
P. Franke and D. Neuschütz: Scientific Group Thermodata Europe (SGTE). Ag-O (Silver - Oxygen). In Binary Systems. Part 5: Binary Systems Supplement 1. Landolt-Börnstein - Group IV Physical Chemistry, vol 19B5. Springer, Berlin
J.L. Brimhall, M.J. Klein, and R.A. Huggins: Acta Metall., 1966, vol. 14, pp. 459–66.
W. Wasserbäch and W. Skrotzki: Mater. Charact., 2023, vol. 195, 112532.
Acknowledgments
This work was financially supported by Nippon Steel Corporation (contract dated 29 June 2016). We are deeply grateful to Prof. Dr. Toshiya Doi, Graduate School of Kyoto University for his help of the sputtering equipment. We thank Mr. Suguru Shiomi and Mr. Keijiro Saito, Graduate School of Kyoto University, for their cooperation in film preparation, Ms. Miyuki Takahashi, Mr. Shuichi Moriwake, Mr. Tetsuya Nagai, and Mr. Tomoya Hiragi of Nippon Steel Technology Co. Ltd. for their careful microstructure analysis, Mr. Yukimasa Ueda and Dr. Junpei Kobata of Osaka Research Institute of Industrial Science and Technology for their precise nanoindentation tests, and Dr. Ryuji Shinya and Mr. Yasuhito Fujishiro of Nippon Steel Technology Co. Ltd. for their advice on thin film analysis.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendices
Appendix 1
O2 flux through an Ag film (JO2) is shown as follows:
where DO is the diffusion coefficient of O in Ag, SO is solubility of O in Ag under PO2 = 1 atm, and symbols of (I) and (II) are inlet and outlet sides of O for Ag films, respectively. We estimated that JO2 > 0.1 Ncc/cm2/min would be required in a large-scale steelmaking plant. To achieve the value of JO2 for an Ag single-crystal film under the conditions of d = 1 μm, PO2(I) = 2.1 atm (pressurized air at 10 atm) and PO2(II) = 1 atm (pure O2 at 1 atm), using Eqs. [A2] and [A3], the film temperature of > 700 °C, i.e., oxygen permeability P (\(={D}_{{\text{O}}}\cdot {S}_{{\text{O}}}\)) of > 3×10-5 Ncc/cm/min/atm0.5 is required.
where the superscript of lat represents lattice of Ag. Values of \({D}_{{\text{O}}}^{{\text{lat}}}\) and \({S}_{{\text{O}}}^{{\text{lat}}}\) are listed in Table AI.
When the Ag film has nanocrystalline structure, the values of D and S can be evaluated as follows:
where fgb is an area fraction of Ag grain boundaries in the cross-section parallel to the Ag film surface, δ is a grain boundary width of Ag, and the superscripts of gb and L represent the grain boundary and the liquid state of Ag, respectively. Here, the grain boundary is assumed to be in the liquid state with a width of three atoms. Values of \({D}_{{\text{O}}}^{{\text{gb}}}\), \({S}_{{\text{O}}}^{{\text{gb}}}\), and δ are listed in Table AI.
To obtain the high value of P > 3×10-5 Ncc/cm/min/atm0.5 corresponding to two thousand times larger than that of single-crystal Ag at a waste gas temperature of 300 °C in a steelmaking plant, fast grain boundary diffusion of Ag films with dg < 50 nm corresponding to fgb > 4 pct is required.
Appendix 2
BF-STEM observations and particle analyses of the cross-section perpendicular to the substrate of the Ag–2 at. pct. Cu–1 at. pct. Al sputtered film before heating (Sample No. 4); full cross-section of the thin film (a), enlarged sub-surface region (b), and EDX analysis (c); fine particles at positions 1 and 2 were identified as those of Al2O3 containing a small amount of CuO; Ga peaks are due to contamination during the FIB sectioning process, and Mo and Si peaks originate from the Mo mesh support and silica substrate, respectively
BF-TEM observations and particle analysis of the cross-section perpendicular to the substrate of the Ag–2 at. pct. Cu–1 at. pct. Al film (Sample No. 4) after heating at 300 °C for 1000 h in air; full cross-section of the thin film (a), enlarged sub-surface region (b), nano-beam electron diffraction pattern with Miller indices at the position of the white circle (c), and EDX analysis (d); the coarse particle indicated by the white arrow was identified as CuO (tenorite) with monoclinic crystal structure
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ueshima, Y., Hasegawa, M., Kubota, N. et al. Thermal Stability of Nanocrystalline Ag–Cu–Al Alloy Films with Densely Dispersed Alumina Particles Prepared via Reactive Sputtering Using Ar–O2 Mixed Gas. Metall Mater Trans A (2024). https://doi.org/10.1007/s11661-024-07392-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11661-024-07392-x