Abstract
Experimental studies of the multicomponent systems are necessary in the development of new materials which can be used in various industries. The literature review showed, that the liquid phase in the silver–gallium–indium system has not been studied yet. In the present work the heat of mixing of the liquid silver–indium and silver–gallium–indium alloys was studied using Calvet type MHTC96 calorimeter. Calorimetric measurements were carried out at the temperatures: 973 K, 1123 K and 1273 K for Ag–In alloys, and at 923 K, 1123 K and 1273 K along two cross-sections XAg/XGa = 1:1 and XGa/XIn = 1:1 for Ag–Ga–In alloys. Next, the Redlich–Kister–Muggianu formalism was applied in the mathematical description of the heat of mixing of the liquid silver–gallium–indium alloys. Experimental results obtained in this work are first, which provided information about the thermodynamic properties of the liquid phase in this ternary system.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The silver–gallium–indium system is used in modern technological applications related to medicine and industry. This ternary system was investigated for the use as electronic skin which can be connected to the human body or as a part of electronic devices.[1,2,3] Electronic skin can be used to monitor health, as a material with self-healing properties or as a component of capsules accelerating the wound healing process.[4] Recently, the novel methods for the production of this electronic skin in the form of a tattoo are developed.[5,6,7] This kind of electronic tattoo can be used in medical devices for monitoring: vital functions, neural connections, level of oxygen, sugar or energy in human body.[8,9,10] Moreover, the ternary Ag–Ga–In system is a candidate for ternary III to V semiconductor nanowires (NWs).[11,12] Taking into account that silver has similar properties but is cheaper than gold, (which is applied in Au-catalysed nanowires), the measurements of Ag-seeded III to V nanowires containing In and Ga can be interesting. Recently, in the literature appeared a description of a process of silver nanowires preparation which were grown in a solution using eutectic gallium–indium (EGaIn) seeds.[13] It is interesting to find out how silver nanostructures can be obtained by applying nontoxic reagents under ambient conditions.
The discovery of recent years are Ag–Cu–In–Ga (ACIG) and (Ag,Cu)(In,Ga)Se2 (ACIGS) materials which can be applied in solar cells.[14,15] These materials are the next generation of Cu(In,Ga)Se2 (CIGS) materials, which are expected to produce film solar cells. The ACIGS materials present less residual stress than CIGS ones, which is an important advantage in the view of flexible photovoltaic applications. The efficiency of ACIGS solar cell under laboratory conditions is about 20 pct and is obtained in a wider temperature range than in the case of CIGS cell.[16,17] In the solar cell preparation process, the control of deposition each element is important. Undeniably, the knowledge about thermodynamic properties and evolution of phase equilibria with temperature change in these materials is required. Moreover, the literature review shown that among lower-order systems which are components of ACIGS materials, the most unknown one is the Ag–Ga–In system.
On the basis of the above information, it can be performed that the new measurements in the silver–indium–gallium system should be conducted. To the best of our knowledge, experimental results of the heat of mixing of the liquid Ag–Ga–In alloys have been presented in this paper for the first time. Finally, the results of this work were described by Redlich–Kister–Muggianu formula.[18]
2 Literature Review
Generally, thermodynamic description of the silver–gallium–indium system is missing in the literature despite of the fact that it will help to understand the optimal conditions the in development of these new materials. The two most popular monographs on phase equilibria in multicomponent systems[19,20] do not contain information about this ternary system as well as its properties. Up to this time, the silver–indium–gallium system was studied only by Campbell and Reynolds.[21] They reported experimental results obtained by applying thermal analysis, photomicrography, X-ray analysis as well hardness testing. These authors presented the phase relations in Ag-rich region in the Ag–Ga–In system at 298 K and also liquidus projection. More experimental results on the Ag–Ga–In system cannot be found in the literature.
Meanwhile, the experimental data and phase equilibria in the Ag–Ga, Ag–In and Ga–In systems which are components of this ternary system were published many times. Recently, the thermodynamic assessments of binary systems: Ag–Ga,[22] Ag–In[23] and Ga–In[24] were published. In them the following information about heat of mixing of these binary system were used.
The heat of mixing of the liquid silver–gallium system has been reported by many researchers who used various types of calorimeters.[25,26,27,28,29,30,31] These results are described in details together with the recent experimental data at 1223 K and 1323 K which were published in our previous paper.[32] All experimental values of the heat of mixing of the liquid Ag–Ga alloys reported in the literature[25,26,29,30,31,32] are negative. It is typical in systems having tendency to intermetallic phase formation. They are also temperature dependent i.e., they become more negative at lower temperature.
In the past, the molar heat of mixing of the liquid silver–indium alloys was examined by many researchers applying calorimetric method. Different techniques were used at different temperatures. Heat of mixing was determined at 723 K using high-temperature calorimeter,[33] at 1243 K using adiabatic calorimeter,[26] at 773 K and 1028 K by applying direct calorimetry[30] and at 743 K and 1280 K[34] using drop calorimetry. Moreover, in work[35] the molar heat of mixing of this liquid alloy was determined at 1100 K. The experimental data reported in cited works have negative values and they agree with one another except for the values given in work[26] which are not so negative like the rest of them. The minimum values of the heat of mixing in the liquid silver–indium alloys are given for different mole fractions of indium at various temperatures: XIn ≅ 0.3 at 1028 K,[30] XIn ≅ 0.4. at 1243 K[26] and 1280 K.[30] In the literature, a dependence on temperature of the molar heat of mixing in the liquid Ag–In alloys is treated in a different manner. In the recent optimisations of Ag–In system Gierlotka[36] and then Muzillo and Anderson[37] accepted temperature dependence of the heat of mixing. Fischer et al.[21] assumed that the heat of mixing is not temperature dependent in this binary system. Taking into account the discrepancies mentioned above, the molar heat of mixing of the liquid silver–indium alloys was studied at 973 K, 1123 K and 1273 K in this work. Values obtained from the experiments will be used in the explanation of these differences. Moreover, they will be used in mathematical description of the molar heat of mixing of the liquid silver–gallium–indium alloys.
The molar heat of mixing of the liquid gallium–indium alloys was examined calorimetrically in the temperature range from 743 to 1423 K.[38,39,40,41,42,43] Reported ΔmixH data are positive for all studied concentrations. It can be assumed that the heat of mixing of liquid Ga–In alloys is temperature independent.
3 Experimental Procedure
The calorimetric studies were carried out in Calvet type MHTC 96 drop calorimeter (Setaram Instruments). This calorimeter and procedure of calorimetric measurements were described in details in previous works.[27,32,43] During our measurements the following materials: silver wire, gallium ingot, indium wire (all 99.999 wt pct, Alfa Aesar, Germany), argon (99.9999 wt pct, Air Products), sapphire rods NIST SRM 720 (99.95 wt pct, NIST) and alumina crucibles (95 wt pct, Powłoka, Poland) were used.
The molar heat of mixing of the liquid silver–indium alloys was studied at 973 K, 1123 K and 1273 K. At the beginning of the measurements, the molar heat of mixing in the liquid silver–indium alloys was determined by adding small pieces of silver (40 to 87 mg), to pure indium bath (approximately 0.50 g). After the addition of silver and standard α-Al2O3, the signal of the recorded heat effect returned to the baseline after 30 minutes. Next, the molar heat of mixing of the liquid ternary Ag–Ga–In alloys was determined at three temperatures 923 K, 1123 K and 1273 K and along two cross-sections XAg/XGa = 1:1 and XGa/XIn = 1:1.The initial value of the molar heat of mixing in the liquid gallium–indium alloys necessary for calculations in this ternary system for chosen composition XIn = 0.5 at 923 K, 1123 K and 1273 K was appointed using parameters given in our work.[43] Next, the initial data of the molar heat of mixing of liquid silver-gallium alloy for chosen composition XAg = 0.5, at 923 K, 1123 K and 1273 K were taken from the literature.[27,32] To ensure data reproducibility, the experiments for cross-section XGa/XIn = 1:1 at 1123 K and 1273 K have been performed two times.
After the end of calorimetric measurements, samples from obtained metallic alloys were studied by optical microscope in order to check dissolution of silver and indium pieces dropped into the bath. Also, selected ternary alloys were tested by X-ray fluorescence method. Total mass of the bath and samples added during experiments differed less than 1 pct from the mass of the obtained alloy after experiments.
4 Results and Discussion
4.1 Experimental Data
The molar heat of mixing in the liquid silver–indium system was measured at 973 K, 1123 K and 1273 K and one experimental run was performed at each temperature. The partial and integral heats of mixing (together with standard uncertainty of the heat of mixing u(ΔmixH)) of liquid silver-indium alloys are gathered in Table I.
The c is the calorimeter constant (JμV−1 s−1) which is calculated from the equation:
Next, the heat of reaction, which was determined in liquid metallic systems, is given by the equation:
finally
where \({n}_{{\mathrm{Al}}_{2}{\mathrm{O}}_{3}}\) is the number of moles of alumina oxide and ni is the number of moles of silver or indium which were introduced into the bath, nb is the number of moles which correspond to amount of bath (indium, silver–gallium or gallium–indium alloy) which was placed in the crucible (all of them given in mol). The TS is the temperature of metals which were introduced into bath, and TM is the measurement temperature (TS and TM are given in K). The \({H}_{{Al}_{2}{O}_{3}}^{{T}_{\mathrm{S}}\to {T}_{\mathrm{M}}}\) and \({H}_{i(i=\mathrm{Ag},\mathrm{ In})}^{{T}_{S}\to {T}_{M}}\) is the heat content of alumina oxide, silver and indium, respectively. It is obtained from reference[44] given in Jmol−1, \({Q}_{{\mathrm{Al}}_{2}{\mathrm{O}}_{3}}\) (μVs) is integral of the measured effect corresponding to the introduction of α-Al2O3 sample into the bath, Qi(i=Ag, In) c (J) is the heat effect corresponding to the introduction of metallic sample into the bath. Next, \(\Delta {H}_{\mathrm{r}}\) (J) is the heat of reaction and \({\Delta }_{\mathrm{mix}}H\) (Jmol−1) is the molar heat of mixing of liquid alloys. The partial heat of mixing (Jmol−1) of liquid alloy is described by the expression:
as a consequence of small masses of metals which were added into the bath.
All values of the heat of mixing obtained at three temperatures in which the liquid silver–indium system was studied are negative. They are shown in Figure 1 and compared with the literature data.[26,30,33,34,35] A visible minimum of the molar heat of mixing of liquid alloys determined at each experimental temperature in this work was found near XIn = 0.30, in agreement with experimental data given in work.[30] It can be seen in Figure 1 that the values from this work agree well with the literature data,[30,33,34,35] except for one series of measurements at 1280 K from work,[34] and they create a consistent description of the liquid phase in this binary system. Experimental values reported in works[26,30] at 1243 K and 1280 K, respectively, are less negative than the values reported in studies[30,33,34,35] as well as in the present work. The molar enthalpy of mixing of liquid silver-indium alloys exhibits temperature dependence in the examined temperature range from 723 to 1273 K.
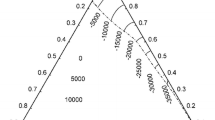
Next, the liquid Ag–Ga–In alloys were studied. The heat of mixing of liquid ternary alloys was measured at 923 K, 1123 K and 1273 K along two cross-sections XAg/XGa = 1:1 and XGa/XIn = 1:1 in eight separate experiments. Obtained experimental results are listed in Tables II and III (together with standard uncertainty of the heat of mixing u(ΔmixH)), and they are shown in Figure 2. The good reproducibility may be observed for the measurements performed along the cross-section XGa/XIn = 1:1 at 1123 K and 1273 K. The starting values of the heat of mixing in liquid silver–gallium system, necessary for the evaluation of results obtained along the cross-section XAg/XGa = 1:1, were taken from two previous works,[27,32] where the heat of mixing in this binary system was described by applying Redlich-Kister polynomial.[45] These experimental values for Ag50Ga50 alloys are following: ΔmixH = − 2730 J mol−1 at 923 K,[27] ΔmixH = − 1923 J mol−1 at 1123 K[27] and ΔmixH = − 1433 J mol−1 at 1273 K.[32] For the cross-section XGa/XIn = 1:1, the heat of mixing in the liquid gallium-indium system required to perform calculations in the liquid ternary system was accepted from previous work.[43] The molar heat of mixing of the liquid gallium-indium system is temperature independent. In this case the value of heat of mixing for Ga50In50 alloys has the same value for each temperature and is equal to ΔmixH = 1143 J mol−1.
The calorimetric data of the molar heat of mixing of the liquid silver–gallium–indium system, obtained along the cross-section XAg/XGa = 1:1 at T = 923 K, 1123 K and 1273 K, show negative values. During the introduction of indium from silver-gallium side, the heat of mixing continuously increases towards pure In. The heat of mixing at 923 K for considered cross-section increases from − 2730 to − 372 J mol−1 for XIn varying from 0 to 0.67, while at 1123 K from about − 1923 to − 229 J mol−1 for XIn increasing from 0 to 0.68. It also increases at 1273 K from − 1433 to − 98 J mol−1 for XIn changing from 0 to 0.68.
Similarly the results of measurements along the cross-section XGa/XIn = 1:1 at T = 923 K, 1123 K and 1273 K, show negative values. Results obtained at 1123 K and 1273 K showed the minimum. The lowest obtained value of the heat of mixing in this work at 923 K is equal − 4819 J mol−1 for XAg = 0.61. The minimum of the heat of mixing at 1123 K is approximately equal to − 4850 J mol−1 for XAg in the range 0.73 to 0.76, and it is approximately equal to − 3540 J mol−1 for XAg from 0.74 to 0.76 at 1273 K. It can be seen that addition of Ag shows strong interaction with indium and gallium resulting in exothermic heat of mixing obtained during experimental measurements in the Ag–Ga–In system along considered cross-section. This is probably connected with the fact that in the binary Ag–Ga and Ag–In systems, a minimum of ΔmixH is located for XAg in the range 0.7 to 0.8. It corresponds to the existence of ζ phase in the solid state in both binary systems.
4.2 Description of Mixing Enthalpy by Applying Mathematical Model
The mathematical description of the molar heat of mixing of liquid silver–gallium, silver–indium and gallium–indium systems was performed using Redlich–Kister polynomial.[45] Parameters for Ag–Ga and Ga–In systems are accepted from works.[32,43] Considering this model, the heat of mixing of the silver–indium system is given by the expression:
Parameters \({}^{v}{W}_{\mathrm{Ag},\mathrm{In}}\) were determined from calculations performed using Thermo-Calc software[46] and are listed in Table IV. In these calculations, calorimetric data of the heat of mixing from this work at 973 K, 1123 K and 1273 K together with the data from works,[30,33,34,35] which were determined in the temperature range from 723 to 1028 K, were applied. Values obtained at 1243 K[26] and at 1280 K[34] are too positive in comparison with the results at 1273 K which are given in our work. Consequently, they were not taken into account in our calculations. The values of the molar heat of mixing of liquid silver-indium system obtained in this work are presented in Figure 1, and they are compared with the literature data together with the results of the model calculations in which Redlich–Kister polynomial[45] was used. The agreement among them is quite good and the heat of mixing of liquid Ag–In system exhibits temperature dependence.
Then, the molar heat of mixing of the liquid Ag–Ga–In system was described by the Redlich–Kister–Muggianu polynomial[18] for substitutional solutions. Considering this model, the heat of mixing of the ternary system is described by the following equation:
Parameters \({}^{v}{W}_{\mathrm{Ag},\mathrm{Ga},\mathrm{In}}\) (enthalpic part of the interaction parameter from R–K–M polynomial) were calculated by applying Thermo-Calc software[46] and are listed in Table IV.
The calculated heat of mixing in the silver-gallium-indium system is plotted in Figure 2, suggesting good agreement with the experimental results obtained in this work. The biggest difference between calculated and experimental values is equal approximately ± 250 J mol-1 at 1123 K for XAg = 0.7493 along XGa/XIn = 1:1 cross-section. The results allow also to draw the conclusion that molar heat of mixing of liquid silver–gallium–indium system exhibits temperature dependence similarly as it was found in the case of the liquid Ag–Ga and Ag–In systems.
Three-dimensional plots of the integral heat of mixing calculated at temperatures: 923 K, 1123 K and 1273 K in the ternary silver–gallium–indium system are shown in Figure 3 together with the experimental results. These figures were created with Statistica 10.0 software.[47] They demonstrate that the minimum of the heat of mixing does not correspond to ternary composition, but it is located on the Ag–In side. It means that the strongest interactions between unlike atoms take place not in ternary but in the binary Ag–In solution.
If one assumes that negative value of the heat of mixing is connected with associates formation in the liquid solution[48,49] these associates should disintegrate with the temperature rise. It may explain the temperature dependence of ΔmixH. Moreover, since the most negative values of the heat of mixing are obtained in the binary Ag–In system, it can be suggested that the formation of the ternary phase is unlikely. The strongest interaction do not take place in ternary solutions. This conjecture is confirmed by the work[24] in which equilibrium diagram of the Ag-rich part of Ag–Ga–In system does not show the presence of ternary phases in the solid state.
The accuracy and correctness of the performed calorimetric measurements was confirmed by the determination of the heat of mixing at the intersection points for studied cross-sections. At these points the measured heat of mixing differs by 90 J mol-1 at 923 K, 70 J mol-1 at 1123 K, and by 40 J mol-1 at 1273 K, respectively. The biggest discrepancy is equal to 90 J mol-1 at 923 K. It allows to conclude that obtained results are reliable.
5 Summary
In this work, using the Calvet type calorimeter, the molar heat of mixing of the liquid silver–indium and silver–gallium–indium alloys was determined between 923 and 1273 K. The obtained results allow to conclude that heat of mixing in this system is temperature dependent. It is important information, which has direct influence on future optimization of the Ag–Ga–In system by applying CALPHAD method, in which evaluation of the Gibbs free energy of phases with temperature change is necessary. Furthermore, the experimental heat data were used to calculate parameters \({}^{v}{W}_{\mathrm{Ag},\mathrm{Ga},\mathrm{In}}\) by application of mathematical model, which in this case was the Redlich–Kister–Muggianu polynomial.[18] The agreement between calculated and experimental results is very good. Finally, it should be pointed out that up to now no information about thermodynamic properties of the liquid phase in this ternary system has been reported in literature.
References
X. Huang, Y. Liu, G.W. Kong, J.H. Seo, Y. Ma, K.I. Jang, J.A. Fan, S. Mao, Q. Chen, D. Li, and H. Liu: Microsyst. Nanoeng., 2016, vol. 2, pp. 16052–60.
S. Xu, Y. Zhang, L. Jia, K.E. Mathewson, K.I. Jang, J. Kim, H. Fu, X. Huang, P. Chava, R. Wang, S. Bhole, L. Wang, Y.J. Na, Y. Guan, M. Flavin, Z. Han, Y. Huang, and J.A. Rogers: Science, 2014, vol. 344, pp. 70–74.
T. Yokota, P. Zalar, M. Kaltenbrunner, H. Jinno, N. Matsuhisa, H. Kitanosako, Y. Tachibana, W. Yukita, M. Koizumi, and T. Someya: Sci. Adv., 2016, vol. 2(e1501856), pp. 1–8.
B.J. Blaiszik, S.L.B. Kramer, M.E. Grady, D.A. McIlroy, J.S. Moore, N.R. Sottos, and S.R. White: Adv. Mater., 2012, vol. 24, pp. 398–01.
P.A. Lopes, H. Paisana, A.T.D. Almeida, C. Majidi, and M. Tavakoli: ACS Appl. Mater. Interfaces, 2018, vol. 10, pp. 38760–68.
Y. Sohn and K. Chu: Mater. Lett., 2020, vol. 265, pp. 127223–226.
P.A. Lopes, D.F. Fernandes, A.F. Silva, D.G. Marques, A.T. Almeida, C. Majidi, and M. Tavakoli: ACS Appl. Mater. Interfaces, 2021, vol. 13, pp. 14552–61.
D.H. Kim, N. Lu, R. Ma, Y.S. Kim, R.H. Kim, S. Wang, J. Wu, S.M. Won, H. Tao, A. Islam, K.J. Yu, T.I. Kim, R. Chowdhury, M. Ying, L. Xu, M. Li, J.H. Chung, H. Keum, M. McCormick, P. Liu, Y.W. Zhang, F.G. Omenetto, Y. Huang, T. Coleman, and J.A. Rogers: Science, 2011, vol. 333, pp. 838–43.
C. Dagdeviren, B.D. Yang, Y. Su, L.P. Tran, P. Joe, E. Anderson, J. Xia, V. Doraiswamy, B. Dehdashti, X. Feng, B. Lu, R. Poston, Z. Khalpey, R. Ghaffari, Y. Huang, M.J. Slepian, and J.A. Rogers: Proc. Natl. Acad. Sci. , 2014, vol. 211, pp. 1927–32.
I.R. Minev, P. Musienko, A. Hirsch, Q. Barraud, N. Wenger, E.M. Moraud, J. Gandar, M. Capogrosso, T. Milekovic, L. Asboth, R.V. Torres, N. Vachicouras, Q. Liu, N. Pavlova, S. Duis, A. Larmagnac, J. Voros, S. Micera, Z. Suo, G. Courtine, and S.P. Lacour: Science, 2015, vol. 347, pp. 159–63.
A.D. Kimberly and P. Caroff: Nanoscale, 2014, vol. 6, pp. 3006–022.
M. Ghasemi, E.D. Leshchenko, and J. Johansson: Nanotechnology, 2021, vol. 32, pp. 072001-1–72020.
C.W. Lin, L.A. Stewart, S. Zhao, G. Akopov, R. Mohammadi, M.T. Yeung, P.S. Weiss, and R.B. Kaner: Z. Anorg. Allg. Chem., 2022, vol. 648, e202200067.
Y. Tauchi, K. Kim, H. Park, and W.N. Shafarman: IEEE J. Photovolt., 2003, vol. 3, pp. 467–71.
S. Soltanmohammad, L. Chen, B. McCandless, and W.N. Shafarman: IEEE J. Photovolt., 2017, vol. 7, pp. 237–80.
S.-C. Yang, J. Sastre, M. Krause, X. Sun, R. Hertwig, M. Ochoa, A.N. Tiwari, and R. Carron: Sol. RRL, 2021, vol. 5, pp. 2100108-1–100116.
S. Essig, S. Paetel, T.M. Friedlmeier, and M. Powalla: J. Phys. Mater., 2021, vol. 4, pp. 024003-1–24009.
Y.J. Muggianu, M. Gambino, and P.J. Bros: J. Chim. Phys., 1975, vol. 22, pp. 83–88.
P. Villars, A. Prince, and H. Okamoto: Handbook of Ternary Alloy Phase Diagrams, ASM International, Metals Park, 1995.
G. Petzow and G. Effenberg: Ternary Alloys A Comprehensive Compendium of Evaluated Constitutional Data and Phase Diagrams, Verlag Chemie, Weinheim, 1988.
A.N. Cambel and W.F. Reynolds: Can. J. Chem., 1962, vol. 40, pp. 37–45.
W. Gierlotka and D. Jendrzejczyk-Handzlik: J. Alloys Compd., 2011, vol. 509, pp. 38–42.
E. Fischer, K. Gajavalli, G. Mikaelian, P. Benigni, J. Rogez, A. Decreton, and M. Barrachin: Calphad, 2019, vol. 64, pp. 292–05.
S.R. Reddy and J.P. Hajra: Calphad, 1993, vol. 17, pp. 151–56.
B. Predel and D.W. Stein: Acta Metall., 1972, vol. 20, pp. 515–22.
K. Itagaki and A. Yazawa: J. Jpn Inst. Met., 1968, vol. 32, pp. 1294–300.
D. Jendrzejczyk-Handzlik and K. Fitzner: J. Chem. Thermodyn., 2011, vol. 43, pp. 392–98.
D. Jendrzejczyk-Handzlik, P. Handzlik, and K. Fitzner: Calphad, 2014, vol. 44, pp. 39–47.
R. Beja ( PhD Thesis), Université Aix-Marseille, 1969.
R. Beja and M. Laffite: C. R. Acad. Sci. Paris C, 1968, vol. 267, pp. 123–26.
D. Li, S. Delsante, W. Gong, and G. Borzone: Thermochim. Acta, 2011, vol. 523, pp. 51–62.
D. Jendrzejczyk-Handzlik: J. Chem. Thermodyn., 2017, vol. 107, pp. 114–25.
O.J. Kleppa: J. Phys. Chem., 1956, vol. 60, pp. 846–52.
R. Castanet, Y. Claire, and M. Laffitte: J. Chim. Phys. Phys.-Chim. Biol., 1970, vol. 67(4), pp. 789–93.
W.A. Badawi and A.M. Oun: Bull. Chem. Soc. Jpn., 1989, vol. 62, pp. 304–08.
W. Gierlotka: J. Electron. Mater., 2012, vol. 41, pp. 86–108.
C.P. Muzillo and T. Anderson: J. Mater. Sci., 2018, vol. 53, pp. 6893–10.
J.P. Bros: C. R. Acad. Sci., 1966, vol. 263, pp. 977–80.
J.P. Bros, R. Castanet, and M. Laffitte: C. R. Acad. Sci., 1967, vol. 264, pp. 1804–06.
B. Predel and D.W. Stein: J. Less-Common Met., 1969, vol. 18, pp. 49–57.
L.A. Mechkovskii and A.A. Vecher: Zh. Fiz. Khim., 1969, vol. 43(5), pp. 1346–48.
I. Ansara, M. Gambino, and J.P. Bros: J. Cryst. Growth, 1976, vol. 32, pp. 101–10.
D. Jendrzejczyk-Handzlik and P. Handzlik: J. Mol. Liq., 2019, vol. 293, 111543.
I. Barin and O. Knacke: Thermochemical Properties of Inorganic Substances, Springer-Verlag, Berlin, 1973.
O. Redlich and A.T. Kister: Ind. Eng. Chem., 1948, vol. 40, pp. 345–48.
J.O. Andersson, T. Helander, L. Höglund, P.F. Shi, and B. Sundman: Calphad, 2002, vol. 26, pp. 273–12.
Statistica 10.0, www.statsoft.com.
F. Sommer: Z. Metallkde., 1982, vol. 2, pp. 73–76.
F. Sommer: Z. Metallkde., 1982, vol. 2, pp. 77–86.
Acknowledgments
This work realized at AGH University of Science and Technology, Faculty of Non-Ferrous Metals under grant number 16.16.180.006 was supported by the Polish Ministry of Education and Science. Authors thank to D. Michalski for his help in experiments.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jendrzejczyk-Handzlik, D., Handzlik, P. Heat of Mixing of the Liquid Silver–Indium and Silver–Gallium–Indium Alloys. Metall Mater Trans A 54, 4744–4756 (2023). https://doi.org/10.1007/s11661-023-07196-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11661-023-07196-5