Abstract
The significance of local equilibria (LE) models in understanding phase transformations in multicomponent steels has been revisited and the necessity for a firsthand computational tool capable of estimating metastable ferrite–austenite and cementite-austenite phase boundaries along with possible LE modes has been realized. For this purpose, thermodynamically consistent Unified Interaction Parameter Formalism (UIPF) has been adopted owing to its computational simplicity. All the necessary parameters required for UIPF-based simulations in Fe–C–Mn system, have been evaluated in the present work. The UIPF-based framework has been shown to be a computationally simple yet an efficient tool for prediction of metastable phase boundaries at low temperatures and wide composition range in the Fe–C–Mn system, where most of the available commercial thermodynamic simulation packages have prediction limitation. The phase boundaries predicted using UIPF have been validated with those predicted by ThermoCalc®, wherever possible/available. Additionally, existing experimental data on growth rates, interlamellar spacing, and partitioning coefficients prevalent during pearlitic transformation in Fe–C–Mn systems have been used to validate the predictions by different LE models adopting UIPF, which in turn affirms the potential of UIPF in predicting thermodynamics and kinetics of phase transformation in Fe–C–Mn systems.




















Similar content being viewed by others
Data Availability
In this work data from ThermoCalc® (commercially available software) has been used for validation purpose. Rest all of the data used/generated in the present work have been explicitly given within the manuscript.
Abbreviations
- \(\alpha\) :
-
Ferrite
- \(\gamma\) :
-
Austenite
- \(\theta\) :
-
Cementite
- \({G}_{\text{m}}^{\phi }\) :
-
Integral molar Gibbs energy of a phase, \(\phi\)
- \({G}_{i}^{0\phi }\) :
-
Standard Gibbs energy of component i in phase \(\phi\), in Raultian standard state
- \({x}_{i}^{\phi }\) :
-
Mole fraction of component i in phase \(\phi\)
- \({G}_{\text{m}}^{E\phi }\) :
-
Integral molar excess Gibbs energy
- \(R\) :
-
Universal gas constant
- \(T\) :
-
Absolute temperature
- \({\overline{G} }_{i}^{\phi }\) :
-
Partial molar Gibbs energy of component i in phase \(\phi\)
- \({\mu }_{i}^{\phi }\) :
-
Chemical potential of component i in phase \(\phi\)
- \({a}_{i}^{\phi }\) :
-
Activity of component i in phase \(\phi\)
- \({\gamma }_{i}^{\phi }\) :
-
Activity coefficient of component i in phase \(\phi\)
- \({\overline{G} }_{i}^{E\phi }\) :
-
Partial molar excess free energy of component i in phase \(\phi\)
- \({\gamma }_{i}^{0}\) :
-
Activity coefficient of component i at infinite dilution
- \({\varepsilon }_{i}^{j\phi }\) :
-
First order interaction parameters
- \({G}_{iH}^{0\phi }\) :
-
Standard Gibbs energy of component i in phase \(\phi\), in Henrian standard state
- \({u}_{\text{M}}\) :
-
U-fraction of substitutional solute M
- \(v\) :
-
Growth rate of pearlite
- \({D}_{C}^{\gamma }\) :
-
Diffusivity of carbon in austenite
- \(S\) :
-
Interlamellar spacing
- \({S}^{\alpha }\) :
-
Thickness of ferrite lamella
- \({S}^{\theta }\) :
-
Thickness of cementite lamella
- \({S}_{C}\) :
-
Critical interlamellar spacing
- \(a\) :
-
Proportionality factor
- \({C}_{i}^{\phi }\) :
-
Concentration in weight percent of component ‘i’ in phase \(\phi\)
- \({C}_{i}^{\gamma /\alpha }\) :
-
Concentration in weight percent of component ‘i’ in phase \(\gamma\) that is in equilibrium with phase \(\alpha\)
- \({C}_{i}^{\gamma /\theta }\) :
-
Concentration in weight percent of component ‘i’ in phase \(\gamma\) that is in equilibrium with phase \(\theta\)
- \(\sigma\) :
-
Interfacial energy between ferrite and cementite
- \({V}_{\text{m}}\) :
-
Molar volume
References
H. Bhadeshia and R. Honeycombe: Steels: Microstructure and Properties, 3rd ed. Butterworth-Heinemann, Oxford, 2006, pp. 53–65.
H.K.D.H. Bhadeshia: Curr. Opin. Solid State Mater. Sci., 2016, vol. 20, pp. 396–400.
Z. Dai, H. Chen, R. Ding, Q. Lu, C. Zhang, Z. Yang, and S. van der Zwaag: Mater. Sci. Eng. R, 2021, vol. 143, 100590.
Z. Dai, X. Wang, J. He, Z. Yang, C. Zhang, and H. Chen: Metall. Mater. Trans. A, 2017, vol. 48A, pp. 3168–174.
Z. Dai, Z. Yang, C. Zhang, and H. Chen: Acta Mater., 2020, vol. 200, pp. 597–607.
N.A. Razik, G.W. Lorimer, and N. Ridley: Acta Metall., 1974, vol. 22, pp. 1249–258.
S.A. Al-Salman, G.W. Lorimer, and N. Ridley: Metall. Trans. A, 1979, vol. 10A, pp. 1703–709.
N.A. Razik, G.W. Lorimer, and N. Ridley: Metall. Trans. A, 1976, vol. 7A, pp. 209–14.
N. Ridley, M.A. Malik, and G.W. Lorimer: Mater. Charact., 1990, vol. 25, pp. 125–41.
S.A. Al-Salman, G.W. Lorimer, and N. Ridley: Acta Metall., 1979, vol. 27, pp. 1391–400.
C. Capdevila, F.G. Caballero, and C. García De Andrés: Acta Mater., 2002, vol. 50, pp. 4629–641.
R.C. Sharma, G.R. Purdy, and J.S. Kirkaldy: Metall. Trans. A, 1979, vol. 10A, pp. 1129–139.
M.M. Aranda, R. Rementeria, J. Poplawsky, E. Urones-Garrote, and C. Capdevila: Scr. Mater., 2015, vol. 104, pp. 67–70.
A.S. Pandit and H.K.D.H. Bhadeshia: Proc. R. Soc. A, 2011, vol. 467, pp. 508–21.
A.S. Pandit and H.K.D.H. Bhadeshia: Proc. R. Soc. A, 2011, vol. 467, pp. 2948–961.
S.K. Tewari and R.C. Sharma: Metall. Trans. A, 1985, vol. 16A, pp. 597–603.
M. Hillert: Scr. Mater., 2002, vol. 46, pp. 447–53.
H.S. Zurob, C.R. Hutchinson, Y. Bréchet, H. Seyedrezai, and G.R. Purdy: Acta Mater., 2009, vol. 57, pp. 2781–792.
M. Gouné, F. Danoix, J. Ågren, Y. Bréchet, C.R. Hutchinson, M. Militzer, G. Purdy, S. Van Der Zwaag, and H. Zurob: Mater. Sci. Eng. R, 2015, vol. 92, pp. 1–38.
C.R. Hutchinson, H.S. Zurob, and Y. Bréchet: Metall. Mater. Trans. A, 2006, vol. 37A, pp. 1711–720.
J.B. Gilmour, G.R. Purdy, and J.S. Kirkaldy: Metall. Trans, 1972, vol. 3, pp. 1455–464.
M. Enomoto, S. Li, Z.N. Yang, C. Zhang, and Z.G. Yang: Calphad, 2018, vol. 61, pp. 116–25.
G. Miyamoto, J.C. Oh, K. Hono, T. Furuhara, and T. Maki: Acta Mater., 2007, vol. 55, pp. 5027–038.
A. Kwiatkowski da Silva, G. Inden, A. Kumar, D. Ponge, B. Gault, and D. Raabe: Acta Mater., 2018, vol. 147, pp. 165–75.
M. Hillert and J. Ågren: Scr. Mater., 2004, vol. 50, pp. 697–699.
M. Hillert and J. Ågren: Scr. Mater., 2005, vol. 52, pp. 87–8.
J. Speer, D.K. Matlock, B.C. De Cooman, and J.G. Schroth: Acta Mater., 2003, vol. 51, pp. 2611–622.
Z. Dai, R. Ding, Z. Yang, C. Zhang, and H. Chen: Acta Mater., 2018, vol. 152, pp. 288–99.
S. Tripathy, P.S.M. Jena, V.K. Sahu, S.K. Sarkar, S. Ahlawat, A. Biswas, B. Mahato, S. Tarafder, and S.G. Chowdhury: Metall. Mater. Trans. A, 2022, vol. 53A, pp. 1806–820.
S. Tripathy, V.K. Sahu, P.S.M. Jena, S. Tarafder, and S.G. Chowdhury: Mater. Sci. Eng. A, 2022, vol. 841, 143034.
G. Miyamoto, H. Usuki, Z.D. Li, and T. Furuhara: Acta Mater., 2010, vol. 58, pp. 4492–502.
C.R. Hutchinson, R.E. Hackenberg, and G.J. Shiflet: Acta Mater., 2004, vol. 52, pp. 3565–585.
H. Guo and M. Enomoto: Metall. Mater. Trans. A, 2007, vol. 38A, pp. 1152–161.
H. Fang, S. van der Zwaag, and N.H. Dijk: Acta Mater., 2021, vol. 212, 116897.
V. Shah, M. Krugla, S.E. Offerman, J. Siestma, and D.N. Hanlon: ISIJ Int., 2020, vol. 60, pp. 1312–323.
G.H. Zhang, R. Wei, M. Enomoto, and D.W. Suh: Metall. Mater. Trans. A, 2012, vol. 43A, pp. 833–42.
C.H.P. Lupis: Chemical Thermodynamics of Materials, Elsevier Science Publishing Co., Inc, New York, 1983, pp. 169–262.
J.O. Andersson, T. Helander, L. Höglund, P. Shi, and B. Sundman: Calphad, 2002, vol. 26, pp. 273–312.
C. Wagner: Thermodynamics of Alloys, Addison-wesley, 1951.
L. Darken: Trans. AIME, 1967, vol. 239, pp. 80–9.
L. Darken: Trans. AIME, 1967, vol. 239, pp. 90–6.
C.W. Bale and A.D. Pelton: Metall. Trans. A, 1990, vol. 21A, pp. 1997–2002.
A.D. Pelton and C.W. Bale: Metall. Trans. A, 1986, vol. 17A, pp. 1211–215.
S. Srikanth and K.T. Jacob: Metall. Trans. B, 1988, vol. 19B, pp. 269–75.
Y.B. Kang: Metall. Mater. Trans. B, 2020, vol. 51B, pp. 795–804.
C.H.P. Lupis: Acta Metall., 1966, vol. 14, pp. 529–38.
C.H.P. Lupis and J.F. Elliott: Acta Metall., 1967, vol. 152, pp. 65–276.
R. David: Gaskell: Introduction to the Thermodynamics of Materials, 4th ed. Taylor & Francis, New York, 2003, pp. 473–78.
M. Hillert: Acta Metall., 1971, vol. 19, pp. 769–78.
M. Hillert: Institute of Metals, London. Monograph No., 1968, vol. 33, pp. 231–47.
Matlab Documentation, https://www.mathworks.com/help/matlab/ref/inv.html.
Octave Docs, https://octave.org/doc/v6.4.0/Solvers.html.
Matlab Documentation, https://www.mathworks.com/help/optim/ug/fsolve.html.
S.H. Lee, K.S. Lee, and K.J. Lee: Mater. Sci. Forum., 2005, vol. 475–479, pp. 3327–330.
A.T. Dinsdale: Calphad, 1991, vol. 15, pp. 317–425.
T. Furuhara, T. Chiba, T. Kaneshita, H. Wu nad G. Miyamoto: Metall. Mater. Trans. A, 2017, vol. 48A, pp. 2739–52.
M.M. Aranda, R. Rementeria, C. Capdevila, and R.E. Hackenberg: Metall. Mater. Trans. A, 2016, vol. 47A, pp. 649–60.
Q. Guo, H. Yen, H. Luo, and S.P. Ringer: Acta Mater., 2022, vol. 225, 117601.
K. Hashiguchi and J.S. Kirkaldy: Scand. J. Metall., 1984, vol. 13, pp. 240–48.
J. Yan, J. Agren, and J. Jeppsson: Metall. Mater. Trans. A, 2020, vol. 51A, pp. 1978–2001.
H. Ishigami, N. Nakada, T. Kochi, and S. Nanba: ISIJ Int., 2019, vol. 59, pp. 1667–675.
T. Tanaka, H.I. Aaronson, and M. Enomoto: Metall. Trans. A, 1995, vol. 26A, pp. 535–45.
Acknowledgments
The authors would like to express their sincere thanks to The Director, CSIR-NML for his permission to carry out this research work under project MLP—3123 and publish it. One of the authors, V.K. Sahu would also like to express sincere gratitude to CSIR HRDG for the financial support (in the form of fellowship).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Appendices
Appendix A. Darken’s Quadratic Formalism
The expression for the logarithm of activity coefficients extended to ternary system by Darken using Quadratic formalism has been given by Equations [A1] through [A3] where \({N}_{2}\), \({N}_{3}\) refer to atom fractions of solutes 2 and 3, while \({\alpha }_{12}\), \({\mathrm{\alpha }}_{13}\), \({\mathrm{\alpha }}_{23}\) are the coefficients of the Quadratic formalism.
Appendix B. Comparison of Model by Lupis and Elliot
Lupis and Elliot expressed the logarithm of activity coefficient of solute and solvent in the form of Taylor series expansion about the point of infinite dilution. The expression in the form of Taylor series expansion sets forth the assumption that all the derivatives of \(ln\gamma\) are finite resulting in an implied assumption that the solute follows Henry’s Law. The Taylor series expansion for \(ln{\gamma }_{1}\), \(ln{\gamma }_{2}\) and \(ln{\gamma }_{3}\) have been given by the Equations [B1] through [B3]. The superscript and subscript of the coefficient ‘\({J}_{i,j}^{k}\)’ represents the solute under consideration (i.e. k) and the order of differential of its logarithm of activity coefficient with respect to solutes (i and j) respectively.
The \({J}_{\mathrm{1,0}}^{(1)}\), \({J}_{\mathrm{1,0}}^{(2)}\) and \({J}_{\mathrm{1,0}}^{(3)}\) terms represent first order differential of logarithm of solvent (1), solute (2) and (3) with respect to solute (2) respectively as given in Equations [B4] through [B6]. Similarly \({J}_{\mathrm{0,1}}^{(1)}\), \({J}_{\mathrm{0,1}}^{(2)}\) and \({J}_{\mathrm{0,1}}^{(3)}\) represent first order differential of logarithm of solvent (1), solute (2) and (3) with respect to solute (3) respectively as given in Equations [B7] through [B9].
The \({J}_{\mathrm{2,0}}^{(1)}\), \({J}_{\mathrm{2,0}}^{(2)}\) and \({J}_{\mathrm{2,0}}^{(3)}\) terms represent the second order differential of logarithm of activity coefficient of solvent (1), solute (2) and (3) with respect to solute (2) respectively as given in Equations [B10] through [B12]. Similarly \({J}_{\mathrm{1,1}}^{(1)}\), \({J}_{\mathrm{1,1}}^{(2)}\) and \({J}_{\mathrm{1,1}}^{(3)}\) represent the second order differential of logarithm of activity coefficient of solvent (1), solute (2) and (3) with respect to solute (2) and (3) respectively, as given in Equations [B13] through [B15]. Furthermore \({J}_{\mathrm{0,2}}^{(1)}\), \({J}_{\mathrm{0,2}}^{(2)}\) and \({J}_{\mathrm{0,2}}^{(3)}\) represent the second order differential of logarithm of activity coefficient of solvent (1), solute (2) and (3) with respect to solute (3) respectively as given in Equations [B16] through [B18].
The coefficient \({J}_{\mathrm{0,0}}^{(2)}\) and \({J}_{\mathrm{0,0}}^{(3)}\) in Equations [B2] and [B3] are the value of \(ln{\gamma }_{2}\) and \(ln{\gamma }_{3}\) at infinite dilution of all solutes, represented by \(ln{\gamma }_{2}^{0}\) and \(ln{\gamma }_{3}^{0}\) respectively (with respect to Raoultian standard state). Further, replacing the \({J}_{i,j}^{k}\) terms in Equations [B2] and [B3] with the interaction parameters (\({\varepsilon }_{2}^{2}\),\({\varepsilon }_{2}^{3}\),\({\varepsilon }_{3}^{2}\), \({\varepsilon }_{3}^{3}{\rho }_{2}^{2}\),\({\rho }_{2}^{\mathrm{2,3}}\),\({\rho }_{2}^{3}\),\({\rho }_{3}^{2}\), \({\rho }_{3}^{3}\) and \({\rho }_{3}^{\mathrm{2,3}}\)) lead to new set of Equations given by Equations [B19] and [B20].
Substituting the expression of \(ln{\gamma }_{1}\), \(ln{\gamma }_{2}\) and \(ln{\gamma }_{3}\) given in Equations [B19] and [B20] in the Gibbs Duhem relation given by Equations [B21] and [B22] yields Equations [B23] and [B24] which can further be simplified resulting in Equations [B25] and [B26]. The term \({J}_{\mathrm{0,0}}^{(1)}\) in the expansion of \(ln{\gamma }_{1}\) is equal to zero (\({\left(ln{\gamma }_{1}\right)}_{{x}_{1\to 1}}=0)\) as the Raoultian standard state has been considered for solvent.
The Equations [B25] and [B26] are of the form
For any value of \({x}_{2}\) and \({x}_{3}\) the expression in B27 will yield a positive value. The only way for the expression in B27 to be zero is that the coefficients A, B, C, D etc. should all be individually zero.
The first order and the second order terms in the expansion of \(ln{\gamma }_{1}\) are obtained only by equating the coefficients A, B and C with zero which yields relations given by Equations [B28] through [B33]. Relation in Equation [B28] implies that the Raoults law is obeyed for the binary system 1– 2.
On substituting the values of \({J}_{\mathrm{0,0}}^{(1)}\), \({J}_{\mathrm{1,0}}^{(1)}\), \({J}_{\mathrm{2,0}}^{(1)}\), \({J}_{\mathrm{1,1}}^{(1)}\) and \({J}_{\mathrm{0,2}}^{(1)}\) in equation [B1] the expression for \(ln{\gamma }_{1}\) can be written as shown in Equation [B34].
Once the series expression for \({\mathrm{ln}}{\gamma }_{1}\) in terms of \({\varepsilon }_{i}^{j}\mathrm{s}\) is obtained, overall excess Gibbs energy of a phase in a ternary system can be evaluated by adopting the expression for integral molar excess Gibbs energy, \({G}_{\text{m}}^{xs}\) as shown in Equation [B35].
Substituting the expression of \(ln{\gamma }_{1}\), \(ln{\gamma }_{2}\) and \(ln{\gamma }_{3}\) in Eq. [B35] yields Eq. [B36].
Equations [B37] through [B39] represent the relationship of partial molar excess Gibbs energy of each component with the integral molar excess Gibbs energy in a ternary system. Adopting Equations [B37] through [B39], expression for \(ln{\gamma }_{1}\), \(ln{\gamma }_{2}\) and \(ln{\gamma }_{3}\) can be evaluated using the second order truncated expansion of integral molar excess Gibbs energy as given by Equation [B36]. These expressions for \(ln{\gamma }_{1}\), \(ln{\gamma }_{2}\) and \(ln{\gamma }_{3}\) are given by Equations [B40] through [B42], which are similar to the ones given by Pelton and Bale for UIPF.
On comparing Equations [B39] and [B40] with Equations [B19] and [B20] respectively, yields the relations given in Equations [B43] through [B45].
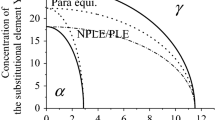
Appendix C. The Compositions Across the Selected Tie–Lines for Austenite-Cementite and Austenite-Ferrite Equilibria Considering PLE and NPLE Mode for Fe–0.8C–1.08Mn and Fe–0.69C–1.8Mn Systems Respectively
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sahu, V.K., Tripathy, S., Chowdhury, S.G. et al. On the Unified Interaction Parameter Formalism and Its Application in Critical Reassessment of Pearlitic Transformation in Fe–C–Mn System. Metall Mater Trans A 54, 3157–3185 (2023). https://doi.org/10.1007/s11661-023-07086-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11661-023-07086-w