Abstract
Selective laser melting (SLM) is one of the promising techniques for producing metallic glass components with unlimited geometries and dimensions. In the case of iron-based metallic glasses, the appearance of cracks remains a problem. In this work, two alloys Fe48Mo14Cr15Y2C15B6 and (Fe0.9Co0.1)76Mo4(P0.45C0.2B0.2Si0.15)20, differing in their plasticity, were printed with a double stage scanning strategy. Both alloys were characterized by a fully amorphous structure and a crack grid that coincided with the hatch distance in the first scan. Segregations of metalloids were observed in the vicinity of the cracks. Fe48Mo14Cr15Y2C15B6 samples were characterized by a high compression strength of 1298 ± 11 MPa and zero plasticity. The compression strength of the (Fe0.9Co0.1)76Mo4(P0.45C0.2B0.2Si0.15)20 samples was 142 ± 22 MPa. The results obtained suggest that further development of scanning strategies and research on the influence of alloying elements is needed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Selective laser melting (SLM) opens the perspective for the production of parts with amorphous structure and complex geometries, unmatched by contemporary bulk metallic glasses (BMGs) production techniques. High cooling rates, which approach 104 to 106 K/s, are more than sufficient for the vitrification of bulk metallic glasses.[1,2] Therefore, the SLM method is suitable for the fabrication of 3D elements, which was experimentally proven for various metallic glass systems: such as Fe-based,[3,4,5] Zr-based,[6,7] Ti-based,[8] and Al-based[9,10] systems. However, in the case of the Fe-based metallic glasses, the obtained parts were far from excellent. Most Fe-based materials exhibit near negligible plasticity at room temperature, which (in combination with the high thermal gradients that occur during laser processing) results in cracking.[11] One proposed mechanism suggests that the cracking is caused by micropores at the edges between the molten pool and the heat affect zone. They lead to stress concentration and, consequently, cracking.[12] The second origin of the cracks could be the annealing caused by the repetitive nature of the SLM process. Annealing above crystallization temperature causes a reduction in free volume, alloy decomposition, and consequently embrittlement of metallic glass.[13,14] The stress intensity factor drops from \(70.4{\text{ MPa}}\sqrt {\text{m}}\) in the as-cast condition to \(3.2{\text{ MPa}}\sqrt {\text{m}}\) in the crystalline regime as well as the fracture strain is reduced from 100 to approximately 0.7 pct.[13] One reason is the appearance of residual tensile stress generated due to the thermal expansion mismatch between the amorphous and nanocrystalline phases.[15] These phenomena combined with high thermal stresses of 1.5 GPa[12] or even 1.8 GPa[16] due to the steep temperature gradient around the molten pool will result in cracking. Especially in the presence of defects, that is, small pores that cause an increase in stress even above 4 GPa.[12] Therefore, crystallized areas, which appear in printed parts, are problematic due to the heterogeneity of the structures and contribution to increased fragility and the crack formation.[17] The crystallites are generally attributed to the high temperature in the heat-affected zone, which is above the transition temperature of the amorphous phase.[18] The content of the crystallized areas can be controlled through different energy inputs,[19] scanning speed,[20] or scanning strategy.[21] These parameters affect heat accumulation in the overlap regions of the layers.[1] Crystallization might also be an effect of incomplete melting of crystalline powder[22] or contaminations present in the powder and during the printing process.[5] However, fully amorphous samples might be obtained by selecting a suitable scanning strategy even for metallic glasses with low glass formation ability.[23] The SLM technique commonly used for thermal stress reduction, such as preheating the substrate or stress-relieving heat treatment, is impractical in the case of metallic glasses because it will cause crystallization. Ye and Shin[24] produced crack-free FeCrMoWMnCSiB samples with direct laser deposition by preheating the build substrate to 422 °C. However, it resulted in the precipitation of a crystalline CrFe phase.[24] Therefore, the adjustment of the scanning strategy seems to be a more perspective solution. Recently, some crack-free samples of Fe-based metallic glasses have been presented, but only for very small samples using a chess board scanning strategy.[25] The other way to limit cracking is to increase the ductility of the feedstock material,[26] for example, zirconia-based metallic glasses are much easier to process without cracking because of the higher ductility.[27]
Guo et al.[11] divided Fe-based metallic glasses into three different categories according to their composition: FeC(B), FeP(C), and Fe(B). The highest ductility and lowest strength characterize those with phosphorus in their composition. This effect was attributed to their lower shear modulus and hence lower shear flow activation energy barrier to shear banding. In consequence, multiple shear bandings are formed in the FeP(C)-based metallic glasses instead of one, single one contributing to larger plastic deformation. To check this concept, two alloys Fe48Mo14Cr15Y2C15B6 and (Fe0.9Co0.1)76Mo4(P0.45C0.2B0.2Si0.15)20 were selected and characterized. Fe48Mo14Cr15Y2C15B6, known as SAM 1651 or SAM7, is characterized by outstanding corrosion and mechanical properties. Its compressive fracture strength is 3200 MPa.[28] Fe48Mo14Cr15Y2C15B6 is commonly used as a coating material deposited by plasma spraying, high-velocity oxygen spraying, or detonation spraying.[29] (Fe0.9Co0.1)76Mo4(P0.45C0.2B0.2Si0.15)20 has high mechanical strength and excellent soft magnetic properties. The ultimate fracture strength and plastic strain reached 3370 MPa and 1.7 pct, respectively.[30] The glass-forming ability measured by the maximum cast diameter was similar for both alloys and amounted to ~ 6 mm.[28,30] In this paper, we compare both alloys in terms of their printability and resulting properties.
2 Experimental Procedure
2.1 Materials
Two selected base materials Fe48Mo14Cr15Y2C15B6 and (Fe0.9Co0.1)76Mo4(P0.45C0.2B0.2Si0.15)20 were gas atomized using industrial raw materials and then sieved to the desired size distribution. The size distribution was measured using a laser diffraction analysis (Anton Paar, PSA1190) and amounted to D10 = 13.82 µm, D50 = 26.69 µm, D90 = 53.69 µm for Fe48Mo14Cr15Y2C15B6 and D10 = 15.17 µm, D50 = 31.57 µm, D90 = 56.83 µm for (Fe0.9Co0.1)76Mo4(P0.45C0.2B0.2Si0.15)20. The cross-sections of manufactured powders are presented in Figure 1.
2.2 SLM Printing
Both alloys were laser melted with a two-stage scanning strategy to maximize glassy phase content[23] at 25 µm powder layer thickness on AISI 316 building plate with Realizer SLM 50 (DMG Mori, Japan) equipped with a 120 W continuous wave 1064 nm fiber laser. First melting, “premelting” was conducted with standard XY scanning strategy at 125 mm/s laser speed, 30 W laser power and hatch distance of 120 µm. Subsequential hatches were scanned in order and a 90 degree rotation was used between layers. Such parameters provided stable melting, and uniform “premelted” material was bonded to the substrate. Second scanning, “remelting” on each layer was conducted at 3000 mm/s laser speed, 120 W laser power, and hatch distance of 30 µm. During the second melting, hatches were scanned in random order to exclude sample overheating and provide uniform heating/cooling rates within the sample. The remelting step was designed to provide a high amorphous phase content and is impossible to realize without premelting because of extensive spatter generation. The samples were created in the form of 15 × 15 × 4 mm chamfered blocks (XYZ) connected directly to the build plate to reduce the risk of delamination and promote heat transfer. The build plate was made of AISI 304 steel.
2.3 Metallographic Examination
The samples for metallographic examination were cut from printed samples and prepared with a standard procedure. The microstructure was then examined with electron scanning microscopes: TESCAN VEGA3 (Tescan, Czech Republic) and ZEISS LEO1455VP (Zeiss,Germany). Nanoscale microstructure studies were carried out with the use of ThermoFisher Themis G2 200 kV analytical electron microscope (STEM) equipped with Ceta™ camera for bright-field observations, Fischione high-angle annular dark-field STEM detector as well as ChemiSTEM™ energy-dispersive spectroscope (EDS) for the analysis of chemical composition. The samples for TEM investigations have been cut out by means of the focused ion beam (FIB) technique with the help of the ThermoFisher Scios 2 Dual Beam microscope equipped with a Ga+ ions source and an EasyLift lift-out system. FIB lamellae were attached to the copper grid with the Pt gas injection system and thinned to ~ 100 nm to secure the transparency of the electron beam in the TEM microscope.
2.4 XRD, DSC, and Oxygen Content Measurement
Phases were determined with the X-ray method using a Co-Kα radiation (Bruker AXS, D8 DISCOVER). Measurement was carried out within the 2θ = 20 to 130 deg with a step size equal to 0.02 deg. The calorimetry of both materials was measured with differential scanning calorimetry (DSC) with a STA 409 C device (Netzsch, Selb, Germany) under an argon atmosphere with a heating rate of 20 K/min. The relative amorphous phase content (Pprinted sample) of the printed samples was measured based on the exothermic enthalpy of the printed sample (ΔHprinted sample) and the amorphous ribbon (ΔHribbon), and it is calculated as Pprinted sample = ΔHprinted sample/ΔHribbon × 100 pct. The relative amorphous phase content of the powder was calculated same. The oxygen content in the feedstock material and printed samples was analyzed with the hot extraction method with gas analyzer (TCHEN 600, Leco Corp.).
2.5 Static Compression Test
The static uniaxial compression test was conducted at ambient temperature on two test series of three specimens each. The tests were based on standards regulations. Rectangular samples with a minimum ratio of 1.6 of principal transverse dimension to height s/h were cut with a wire electrical discharge machine (EDM). The contact surfaces of the specimens were machined to obtain low roughness and parallelism.In addition, an articulated joint was applied to compensate for the bending moment and a compression-centered test piece. The compression test was carried out on an Instron 5982 test system at a constant strain rate of 1 ×·10–4 s−1 until fracture. In addition to compression characteristics, the basic strength properties of the samples belonging to both material groups were determined: proportional limit (σ0.05), compressive offset yield strength (σ0.2—as a conventional quantity defining the stress value corresponding to permanent deformation of the specimen measurement base h by 0.2 pct and adopted for comparison), compressive strength (σ), and relative shortening of the sample length indicating the material's ability to deform in compression (aC—determined from compression curves).
2.6 Nanoindentation
Vickers microhardness measurements were performed with a load of 0.98 N (equal to 0.1kgf) and a Wilson Tukon 1102 device. Additionally, the nanoindentation was performed with a Berkovich indent and the following parameters: a load of 50 mN, a loading rate of 100 mN/min, and a dwell time of 30 seconds (Anton Paar, NHT 3). Ten indents were performed for each sample. The fracture toughness Kc of the printed samples was estimated from the indentation results based on the energy method described in References 31, 32.
3 Results and discussion
3.1 XRD
Both powders and printed samples exhibit broad halo peaks that confirm their amorphous nature (Figure 2). The alloy Fe48Mo14Cr15Y2C15B6 might be considered entirely amorphous both in the case of powder and printed samples. In the case of (Fe0.9Co0.1)76Mo4(P0.45C0.2B0.2Si0.15)20 alloy powder contains some crystallization peaks in the XRD patterns. They have been identified as α-iron. These crystalline peaks disappeared after the SLM printing process and the printed sample is fully amorphous. Such a counterintuitive behavior of the alloys suggests that the heating and cooling rates for SLM with the proper strategy are higher than those for gas atomization. The data obtained partially contradict literature data,[28,30] suggesting a similar glass-forming ability for both alloys, as the alloy (Fe0.9Co0.1)76Mo4(P0.45C0.2B0.2Si0.15)20 underwent partial crystallization during the atomization process, even for relatively small powder diameters in the range of + 15 to 50 µm used in this study.
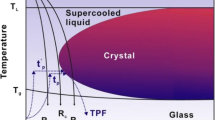
Crystallization of metallic glasses might be estimated based on time–temperature–transformation (TTT) diagrams, which describe the thermal stability of BMGs. Crystallization during 3D printing will occur when the once thermal cycle curves intersect with the TTT diagrams.[33] The Fe48Mo14Cr15Y2C15B6 alloy was found to have an amorphous structure for cooling rates below 190 K/s and a mixed structure with crystalline phase M23C6 type for cooling rates between 140 and 190 K/s.[34] Additionally, the reported crystallization behavior of Fe48Mo14Cr15Y2C15B6 is similar in both heating and cooling as opposed to Zr-based metallic glasses which require faster heating than cooling rates to prevent crystallization.[35,36] To maintain sufficient cooling rates and prevent crystallization, the hatches in this study during the remelting stage (as escribed in the experimental part) were separated. A random scanning sequence was applied instead of scanning one hatch after another.[23] This allowed the heat to dissipate before the next laser scan and in consequence prevented heat accumulation and secondary devitrification of the alloy.
3.2 DSC
The results of the DSC investigation are presented in Figure 3. For Fe48Mo14Cr15Y2C15B6, the relative content of amorphous phase amounts to 85.3 pct for powder and 81.7 pct for printed sample. For (Fe0.9Co0.1)76Mo4(P0.45C0.2B0.2Si0.15)20 the relative content of the amorphous phase amounts to 91.4 pct for powder and 90.5 pct for printed sample. Fe48Mo14Cr15Y2C15B6 exhibits three exothermic peaks. Crystallization in this alloy starts at around 617 °C (crystallization temperature Tx) for ribbon. (Fe0.9Co0.1)76Mo4(P0.45C0.2B0.2Si0.15)20 exhibit four exothermic peaks for the ribbon and three exothermic peaks for the printed sample and powder. The onset of the first is at 470 °C (Tx) for the ribbon. The results show that, despite the higher crystallization temperature, the Fe48Mo14Cr15Y2C15B6 alloy has undergone more relaxation and contains more nanocrystallines than (Fe0.9Co0.1)76Mo4(P0.45C0.2B0.2Si0.15)20, which was not captured by XRD. This might be caused by a higher oxygen intake described in the next paragraph.
3.3 Oxygen Content
The oxygen content in (Fe0.9Co0.1)76Mo4(P0.45C0.2B0.2Si0.15)20 alloy amounted to 0.031 ± 0.006 pct for feedstock powder and 0.04 ± 0.006 pct for printed samples. In the case of Fe48Mo14Cr15Y2C15B6 alloy, the oxygen content was 0.12 ± 0.02 pct for powder and 0.08 ± 0.006 pct for printed samples. The higher oxygen content in Fe48Mo14Cr15Y2C15B6 powder is a consequence of the powder shape, which is not perfectly spherical. The grains are elongated, resulting in a higher specific surface area and thus higher oxygen intake. The higher oxygen content in the feedstock powder means a higher powder content in the printed samples, and this is twice as high as for the (Fe0.9Co0.1)76Mo4(P0.45C0.2B0.2Si0.15)20 alloy. The oxygen content is critical for the properties of metallic glasses, because it related to the increase in the tendency to crystallize and deterioration of the fracture toughness. According to the study by Idury et al.[37] study the O content up to 3500 ppm has a marginal influence on both the plastic strain and the compressive yield. The values of 800 ppm for Fe48Mo14Cr15Y2C15B6 and 400 ppm for (Fe0.9Co0.1)76Mo4(P0.45C0.2B0.2Si0.15)20 are much below this threshold value, thus could be seen as acceptable.
3.4 Microstructure
The microstructures of the printed samples are shown in Figure 4. Microscopic observation did not show the presence of the crystallization phase both for the Fe48Mo14Cr15Y2C15B6 and (Fe0.9Co0.1)76Mo4(P0.45C0.2B0.2Si0.15)20. The Fe48Mo14Cr15Y2C15B6 and (Fe0.9Co0.1)76Mo4(P0.45C0.2B0.2Si0.15)20 samples have repeating crack patterns throughout the structure. Depending on the side of the samples observed by microscopy, the cracks were observed as parallel lines or as a square grid. However, (Fe0.9Co0.1)76Mo4(P0.45C0.2B0.2Si0.15)20 showed a higher crack density and the presence of pores along the cracks. Furthermore, the crack grid in (Fe0.9Co0.1)76Mo4(P0.45C0.2B0.2Si0.15)20 was less regular (less square, more round) than the cracks in the structure of Fe48Mo14Cr15Y2C15B6. The fracture pattern in the form of straight lines shows that the Fe48Mo14Cr15Y2C15B6 alloy is a typical brittle material. Large pores (holes) were also observed in Fe54.35Cr18.47Mn2.05Mo13.93W5.77B3.22C0.90Si1.32 in work[38] when the density of laser energy was not enough to melt the feedstock powder. The not fully melted feedstock led to low-density samples and pores in which crakes initiated due to the presence of residual stress in the sample. However, not fully melting the feedstock is not a proper explanation for the alloys in this work, especially for (Fe0.9Co0.1)76Mo4(P0.45C0.2B0.2Si0.15)20 because the XRD of the BMG shows the annihilation of the crystal phase that was observed in a used powder feedstock. This indicates that the melting of the feedstock was achieved successfully. However, the cracks are arranged in a grid or strip pattern (depending on the observation site) with an interval of approximately 120 µm in both alloys. This is most evident in (Fe0.9Co0.1)76Mo4(P0.45C0.2B0.2Si0.15)20. The crack pattern corresponds to the original scanning strategy, where the cracks go according to the hatch distance. This means that the initial cracks were not removed by remelting in the second pass or were only partially eliminated. The existing cracks outside the remelting zone then propagated through the remelted spot, thus retaining its original grid layout. For the Fe48Mo14Cr15Y2C15B6 alloy, some cracks either did not form or were partially eliminated. There is a greater distance between the cracks, although in some places the cracks are becoming more dense. Therefore, random scanning for amorphization fails to eliminate the existing cracks in the material. The overlap of the cracks with the hatch indicates that the crack occurred at the boundary of the fusion line. This may be the result of a combination of several phenomena. Low laser power with a wide hatch causes insufficient fusion with the previous track, similarly to the one described in Reference 39. The other might be annealing as a result of elevated temperature. The metallic glass becomes brittle during annealing close to Tg, even before the onset of crystallization.[40] During the printing process, the material is cyclically affected by temperature, which causes annealing and structure relaxation. In Reference 41 researchers proposed a parameter called Tcf (critical fictive temperature). The critical fictive temperature is characteristic of the BMG former denoting the ductile–brittle transition, which together with Tf (fictive temperature) might be used to assess the room temperature plasticity of metallic glasses. For most Fe-based BMGs, Tf is lower than Tfc (critical fictive temperature); therefore, their plasticity is strongly dependent on the cooling rates; in case of too slow cooling, they become brittle.[41] Garrett et al.[42] suggested that fragile glass formers would be more prone to annealing embrittlement compared to stronger glass formers and, as such, their fracture toughness would be more sensitive to their processing history. In Reference 43, the Cu-based BMG obtained by the SLM process showed higher nanohardness and elastic modules than the same alloy obtained by casting, which was prescribed to the structure relaxation. However, the drop in fracture resistance below crystallization temperature is not always associated with a change in hardness or modulus values.[13] An increase in brittleness was also observed due to structural segregation linked to irreversible microsegregation.[44] As can be seen, the thermal influence in the SLM process causes many changes in the BMG structures even below the Tg. Relaxation and segregation of the structure make a BMG more brittle. Additionally, the cooling rates might be too low to obtain the plasticity of Fe-based metallic glasses. It should be noted that the high heating and cooling rate in SLM shifts the rate of the transient nucleation process by up to 15 orders below the steady-state nucleation rate.[45] Therefore, even if the crystallization is not visible in the printed samples, the temperature could have achieved Tg or a temperature close to below in the heat-affected zone (HAZ). Paradoxically, if crystallization had occurred, the printed samples could exhibit more plasticity. Li et al.[15] showed that it is possible to preserve a certain ductility in two cases: (I) large amorphous film thickness with low hydrostatic pressure and (II) tiny amorphous film thickness and high hydrostatic pressure. Therefore, increasing the volume fraction of nanocrystals above 90 pct, while maintaining their small size, is beneficial.[15] The other strategy to reduce cracking is adjusting the scanning strategy. Zou et al.[25] tested the influence of chessboard scanning strategy with the size of the single square of the cheeseboard is smaller than the critical crack length. The residual stress of the samples printed by the chessboard scanning strategy was reduced compared to that of the straight-line scanning strategy. The cracks were not observed, but it should be mentioned that the samples have only 0.1 mm of thickness. In Reference 25, ED (energy density) is quite high (90.9 J/mm3) and is the same in both meltings. In Reference 9, the second remelting performed with lower ED provides release of the accumulated stress after the first metling and consequently reduces the number of crakes, which was also observed in Fe48Mo14Cr15Y2C15B6 in this work. However, in this work, the ED in remelting was done not by decreasing the laser power but by increasing the scanning speed and significantly increasing the laser power. This different approach to decreasing ED should be studied in further research. The comparison of parameters used is given in the Table I.
Another idea to reduce the propagation of cracks in the printed samples is to create in situ or ex situ BMG composites where the glassy matrix is reinforced with very ductile crystal particles.[35,46] In both cases, the appropriate parameters allow to a create proper connection between the glassy phase and particles. The connection leads to the transfer of load from the brittle matrix to the ductile phase. In the ductile phase, shear bands are multiple and delay the propagation of cracks. However, the enhanced plasticity of these composites by creating ductile particles reduced the compressive stress of the samples. In Reference 47, adding 0.5 at. pct of Cu to Fe-based BMG to form an in situ composite reduced compressive strength by 17 pct (4511 MPa BMG, 3755 MPa BMG reinforced with 0.5 pct Cu particles) while increasing the plastic strain 3 times (1.5 pct BMG, 4.5 pct BMG with 0.5 pct Cu). Another example of a Fe-based metallic glass composite is Fe77Mo5P9C7.5B1.5 BMG reinforced by in situ formed ductile α-Fe dendrites.[48] It has a plastic strain of more than 30 pct and a high fracture strength of more than 3.0 GPa.[48]
The further discussed mechanism that contributes to the brittleness of Fe-based metallic glasses is the formation of inhomogeneities due to the start of crystallization.[13] In both samples, a strong segregation of metalloids is visible in cracks, especially carbon and boron (Figures 5 and 6). This contributes to the stress concentration and hence crack formation. Similar carbon segregation was observed by Zou et al.[49] Chemical elements with small radius are known to segregate in metallic glasses during annealing to the grain boundaries.[15] Therefore, it can be assumed that, in the case of fabricated samples, these elements will move into the vicinity of the HAZ, contributing to an increased embrittlement at this location, already affected by other mechanisms. Furthermore, FeP-based metallic glass systems are prone to spinodal decomposition and brittleness caused by phosphorus segregation.[50] The crack tip propagates then along the fracture planes with a higher concentration of P.[50] In the alloy (Fe0.9Co0.1)76Mo4(P0.45C0.2B0.2Si0.15)20, the segregation of phosphorus is less apparent, but the mechanism is probably also valid for carbon and boron. In Reference 51, carbon and boron were found to be likely to co-segregate in grain boundaries, dislocation, or vacancy in ferrite or martensite steels, while in Reference 52, it was proven that boron segregation in austenitic boundaries suppresses the phosphor segregation. Similar phenomena can occur in observed (Fe0.9Co0.1)76Mo4(P0.45C0.2B0.2Si0.15)20 BMG, causing suppression of P segregation and co-segregation of the C and B.
3.5 Nanoindentation and Hardness Measurements
The data obtained from the nanoindentation and hardness measurements for both alloys are presented in Table II. The values of the hardness and the indentation elastic module are lower for (Fe0.9Co0.1)76Mo4(P0.45C0.2B0.2Si0.15)20 than for Fe48Mo14Cr15Y2C15B6, which supports its higher plasticity. The obtained value for Fe48Mo14Cr15Y2C15B6 of 226 GPa is higher than the 206 GPa given in the literature[53] for samples cast from the same alloy. There is a lack of data for (Fe0.9Co0.1)76Mo4(P0.45C0.2B0.2Si0.15)20 but the measured value of 177 GPa is similar to 179 GPa reported by Zhang et al. for Fe70Mo5P10C10B5.[54] The increase in modulus usually indicates a decrease in the atomic distance that is related to the loss of free volume.[55] Therefore, Young’s modulus increases even without XRD-detectable crystallization.[56] The increase in Young’s modulus in this research is in line with these phenomena. The heat delivered during the SLM process resulted in a loss of free volume and possibly some nanoscale heterogeneity regions. The HVIT values from nanoindentation are slightly higher than HV0.1, due to much lower load. The decrease in hardness with increased load is called the indentation size effect (ISE). It is observed in both crystallite and amorphous materials.[57] In crystallite materials, this effect is related to loss of elastic recovery and the beginning of plastic deformation. In the shallow indentations, the crystal lattice becomes distorted, and additional dislocations have to be created to account for the large shear strains. Large penetration depths result in more gradual shear strain variation, which can be accommodated by statistically stored dislocations. The other contributing mechanism is the evolution of damage.[58] In metallic glasses, the dislocations cannot be formed; therefore, the indentation size effect is prescribed to deformation-induced excess of free volume.[59] The indentation size effect is weaker in metallic glasses than in crystallized materials.[60] The largest decrease in hardness with depth occurs at small depths; as depth increases, the changes are smaller, the correlation plot flattens out.[57,60] In light of the relationships presented, the decrease in measured hardness seems plausible. In general, the obtained data are comparable with those reported with the literature and confirm the amorphous structure of the printed samples.[28,61]
The estimated value of fracture toughness (Table II) is low and similar to that given in the literature for Fe-based metallic, for example, 1.25 to 2.05 MPa m1/2 for Fe47-xCr20Mo10WxC15B6Y2,[62] 5.5 MPa m1/2 for Cr25-27, Mo16-18, C2.0 to 2.5, B2.0 to 2.2, Fe balance (wt pct),[63] 4.8 − 6.0 MPa m1/2 for Fe77−x−yCrxMoyC15B6Y2 (atom pct).[53] A slightly higher value is recorded for (Fe0.9Co0.1)76Mo4(P0.45C0.2B0.2Si0.15)20 than for Fe48Mo14Cr15Y2C15B6 but with much higher scatter. It could be prescribed to higher plasticity of (Fe0.9Co0.1)76Mo4(P0.45C0.2B0.2Si0.15)20 and a lower oxygen content in the printed sample. The high scatter might be caused by a lower crystallization temperature of this alloy and hence a greater number of areas affected by thermal cycling caused by the SLM process. Despite the higher fracture strength, the printability of (Fe0.9Co0.1)76Mo4(P0.45C0.2B0.2Si0.15)20 is worse.
3.6 Static Compression Test
The samples for testing were cut in C according to the scheme in Figure 4. Due to the numerous cracks present in the samples, the tests are comparative in nature. The results are presented in the Table III. Both studied materials show elastic-brittle behavior (Figure 7). In particular, the Fe48Mo14Cr15Y2C15B6 alloy is characterized by a compression curve similar to that of glasses, characterized by an unspecified relative shortening, while having high compressive strength with very low scatter. The obtained compressive strength of 1298 MPa is ~ 40 pct of the compressive strength of the casted samples.[28]
In the case of the second alloy (Fe0.9Co0.1)76Mo4(P0.45C0.2B0.2Si0.15)20, the mechanical properties are very low (Table III). The compression strength reached 142 MPa, which is only 4 pct of the value for casted samples.[30] Despite the low mechanical properties, there is still some plastic strain. Its value is close to that reported in the literature.[30] Based on this phenomenon, it should be expected that the printability of (Fe0.9Co0.1)76Mo4(P0.45C0.2B0.2Si0.15)20 will be slightly better, and the density of the cracks will decrease at least slightly compared to Fe48Mo14Cr15Y2C15B6. However, it was not confirmed in the microstructural observations. The (Fe0.9Co0.1)76Mo4(P0.45C0.2B0.2Si0.15)20 exhibited a much higher crack density with a smaller spacing.
The surface of the samples after the static compression test is presented in the Figure 8. The fracture surface of the sample Fe48Mo14Cr15Y2C15B6 and (Fe0.9Co0.1)76Mo4(P0.45C0.2B0.2Si0.15)20 did not present the vain pattern that is typical for ductile BMGs. There is also no distinct one shear plane. The fracture surface is smooth with some mist and hackle regions. Therefore, it could be stated that both alloys represent brittle cleavage fracture, which is in agreement with the fracture characteristics of brittle metallic glasses.[64] Furthermore, in (Fe0.9Co0.1)76Mo4(P0.45C0.2B0.2Si0.15)20, also the Wallner lines can be observed. These lines occurred as an interaction between the propagation of the crack and the shear wave.[65] This phenomenon is mostly observed in the brittle BMG. The defects present in the samples contribute to the increase in the number of fracture sites. This is especially visible in the case of (Fe0.9Co0.1)76Mo4(P0.45C0.2B0.2Si0.15)20, where there are some separate cuboids formed along the cracks. This explains the very low strength of this sample. The reason for the lack of sintering of the alloy (Fe0.9Co0.1)76Mo4(P0.45C0.2B0.2Si0.15)20 compared to the alloy Fe48Mo14Cr15Y2C15B6 with the same parameters is not entirely clear and requires further studies. Probably because of the lower Tg temperature, this material becomes more brittle in the HAZ zone.
4 Conclusions
This work demonstrated the possibility of preparing fully amorphous structures with a two-stage scanning strategy using amorphous Fe48Mo14Cr15Y2C15B6 and partially crystallized (Fe0.9Co0.1)76Mo4(P0.45C0.2B0.2Si0.15)20 powder. Samples from both alloys were characterized by a crack grid that coincided with the hatch spacing of the initial “premelting” strategy. These cracks were not eliminated by second scanning. Probably the cracks present in the surrounded area after first melting were able to propagate through remelted regions; therefore, their initial arrangement has been retained. Crack formation was promoted by the segregation of elements present in the composition of iron-based metallic glass alloys, particularly boron and carbon. No positive effect of using (Fe0.9Co0.1)76Mo4(P0.45C0.2B0.2Si0.15)20 alloy with some ductility was observed. On the contrary, this alloy was characterized by worse properties in terms of microstructure than less ductile Fe48Mo14Cr15Y2C15B6 alloy. The reason could be a lower crystallization temperature as well as the presence of phosphorus in the composition, which usually worsens the weldability of steel. Further work (including TEM investigation) is required to relate alloy chemical composition to cracking susceptibility and glass-forming ability to cracking susceptibility. At the same time, the high values of mechanical properties (compression strength equal to 1298 ± 11 MPa) obtained in the case of the alloy Fe48Mo14Cr15Y2C15B6, despite the presence of numerous cracks, should be emphasized. Thus, it appears that the SLM process is a promising method for producing parts with excellent mechanical properties from Fe-base metallic glasses; however, further development of scanning strategies and research on the influence of alloying elements are needed.
References
D. Ouyang, W. Xing, N. Li, Y. Li, and L. Liu: Addit. Manuf., 2018, vol. 23, pp. 246–52.
S. Katakam, J.Y. Hwang, S. Paital, R. Banerjee, H. Vora, and N.B. Dahotre: Metall. Mater. Trans. A, 2012, vol. 43A, pp. 4957–66.
H.Y. Jung, S.J. Choi, K.G. Prashanth, M. Stoica, S. Scudino, S. Yi, U. Kühn, D.H. Kim, K.B. Kim, and J. Eckert: Mater. Des., 2015, vol. 86, pp. 703–08.
N. Li, J. Zhang, W. Xing, D. Ouyang, and L. Liu: Mater. Des., 2018, vol. 143, pp. 285–96.
S. Pauly, L. Löber, R. Petters, M. Stoica, S. Scudino, U. Kühn, and J. Eckert: Mater. Today, 2013, vol. 16, pp. 37–41.
D. Ouyang, N. Li, W. Xing, J. Zhang, and L. Liu: Intermetallics, 2017, vol. 90, pp. 128–34.
S. Pauly, C. Schricker, S. Scudino, L. Deng, and U. Kühn: Mater. Des., 2017, vol. 135, pp. 133–41.
L. Deng, S. Wang, P. Wang, U. Kühn, and S. Pauly: Mater. Lett., 2018, vol. 212, pp. 346–49.
X.P. Li, C.W. Kang, H. Huang, and T.B. Sercombe: Mater. Des., 2014, vol. 63, pp. 407–11.
X.P. Li, M.P. Roberts, S. O’Keeffe, and T.B. Sercombe: Mater. Des., 2016, vol. 112, pp. 217–26.
S.F. Guo, J.L. Qiu, P. Yu, S.H. Xie, and W. Chen: Appl. Phys. Lett., 2014, vol. 105, p. 161901.
W. Xing, D. Ouyang, N. Li, and L. Liu: Intermetallics, 2018, vol. 103, pp. 101–06.
C. Minnert, M. Kuhnt, S. Bruns, A. Marshal, K.G. Pradeep, M. Marsilius, E. Bruder, and K. Durst: Mater. Des., 2018, vol. 156, pp. 252–61.
R. Gerling, F.P. Schimansky, and R. Wagner: Acta Metall., 1988, vol. 36, pp. 575–83.
F. Li, T. Liu, T. Wang, A. Wang, J. Wang, and Y. Yang: J. Mech. Phys. Solids, 2019, vol. 132, p. 103681.
D. Ouyang, Q. Zheng, L. Wang, H. Wang, C. Yang, P. Zhang, and N. Li: Mater. Sci. Eng. A, 2020, vol. 782, p. 139259.
Y. Zhang, X. Lin, L. Wang, L. Wei, F. Liu, and W. Huang: Intermetallics, 2015, vol. 66, pp. 22–30.
G. Yang, X. Lin, F. Liu, Q. Hu, L. Ma, J. Li, and W. Huang: Intermetallics, 2012, vol. 22, pp. 110–15.
X.P. Li, C.W. Kang, H. Huang, L.C. Zhang, and T.B. Sercombe: Mater. Sci. Eng. A, 2014, vol. 606, pp. 370–79.
Y. Shen, Y. Li, and H.-L. Tsai: J. Non-Cryst. Solids, 2018, vol. 481, pp. 299–305.
Ł. Żrodowski, B. Wysocki, R. Wróblewski, K.J. Kurzydłowski, W. Święszkowski: Fraunhofer Direct Conference Proceedings of Digital Manufacturing Conference DDMC, vol. 2016.
V.K. Balla and A. Bandyopadhyay: Surf. Coat. Technol., 2010, vol. 205, pp. 2661–67.
Ł Żrodowski, B. Wysocki, R. Wróblewski, A. Krawczyńska, B. Adamczyk-Cieślak, J. Zdunek, P. Błyskun, J. Ferenc, M. Leonowicz, and W. Święszkowski: J. Alloy Compd., 2019, vol. 771, pp. 769–76.
X. Ye and Y.C. Shin: Surf. Coat. Technol., 2014, vol. 239, pp. 34–40.
Y. Zou, Y.S. Wu, K. Li, C.L. Tan, Z.G. Qiu, and D.C. Zeng: Mater. Lett., 2020, vol. 272, p. 127824.
Z. Mahbooba, L. Thorsson, M. Unosson, P. Skoglund, H. West, T. Horn, C. Rock, E. Vogli, and O. Harrysson: Appl. Mater. Today, 2018, vol. 11, pp. 264–69.
Y.H. Liu, G. Wang, R.J. Wang, D.Q. Zhao, M.X. Pan, and W.H. Wang: Science, 2007, vol. 315, pp. 1385–88.
J. Shen, Q. Chen, J. Sun, H. Fan, and G. Wang: Appl. Phys. Lett., 2005, vol. 86, p. 151907.
L. Xie, Y.-M. Wang, X. Xiong, and Z.-K. Chen: Mater. Trans., 2018, vol. 59, pp. 1591–95.
F. Li, B. Shen, A. Makino, and A. Inoue: Appl. Phys. Lett., 2007, vol. 91, p. 234101.
A.B. Behboud, A. Motallebzadeh, and S. Özerinç: J. Alloy Compd., 2022, vol. 901, p. 163578.
J. Chen: J. Phys. D, 2012, vol. 45, p. 203001.
Y. Lu, H. Zhang, H. Li, H. Xu, G. Huang, Z. Qin, and X. Lu: J. Non-Cryst. Solids, 2017, vol. 461, pp. 12–17.
K. Hildal, N. Sekido, and J.H. Perepezko: Intermetallics, 2006, vol. 14, pp. 898–902.
W. Hu, Z. Yu, Y. Lu, J. Huo, Z. Qin, X. Lu, and R.L. Narayan: J. Alloy Compd., 2022, vol. 918, p. 165539.
D.V. Louzguine-Luzgin, A.I. Bazlov, S.V. Ketov, A.L. Greer, and A. Inoue: Acta Mater., 2015, vol. 82, pp. 396–402.
K.S.N.S. Idury and R.L. Narayan: Trans. Indian Inst. Met., 2022.
X.D. Nong, X.L. Zhou, and Y.X. Ren: Opt. Laser Technol., 2019, vol. 109, pp. 20–26.
N.T. Aboulkhair, N.M. Everitt, I. Ashcroft, and C. Tuck: Addit. Manuf., 2014, vol. 1–4, pp. 77–86.
P. Murali and U. Ramamurty: Acta Mater., 2005, vol. 53, pp. 1467–78.
G. Kumar, P. Neibecker, Y.H. Liu, and J. Schroers: Nat. Commun., 2013, vol. 4, p. 1536.
G.R. Garrett, M.D. Demetriou, M.E. Launey, and W.L. Johnson: Proc. Natl. Acad. Sci., 2016, vol. 113, pp. 10257–62.
X. Lu, M. Nursulton, Y. Du, and W. Liao: Materials, 2019, vol. 12, p. 775.
D. Rajpoot, R.L. Narayan, L. Zhang, P. Kumar, H. Zhang, P. Tandaiya, and U. Ramamurty: J. Mater. Sci. Technol., 2022, vol. 106, pp. 225–35.
A. Ericsson, V. Pacheco, M. Sahlberg, J. Lindwall, H. Hallberg, and M. Fisk: Mater. Des., 2020, vol. 195, p. 108958.
S. Guo, J. Wang, H. Zhang, and S. Xie: Trans. Nonferr. Met. Soc. China, 2012, vol. 22, pp. 348–53.
J.M. Park, D.H. Kim, and J. Eckert: J. Alloy Compd., 2012, vol. 536, pp. S70-73.
S.F. Guo, L. Liu, N. Li, and Y. Li: Scripta Mater., 2010, vol. 62, pp. 329–32.
Y. Zou, Z. Qiu, C. Tan, Y. Wu, K. Li, and D. Zeng: J. Non-Cryst. Solids, 2020, vol. 538, p. 120046.
Y. He, P. Yi, and M.L. Falk: Phys. Rev. Lett., 2019, vol. 122, p. 35501.
G. Da Rosa, P. Maugis, J. Drillet, V. Hebert, and K. Hoummada: J. Alloy Compd., 2017, vol. 724, pp. 1143–48.
S. Suzuki, M. Tanino, and Y. Waseda: ISIJ Int., 2002, vol. 42, pp. 676–78.
Y.-C. Li, C. Zhang, W. Xing, S. Guo, and L. Liu: ACS Appl. Mater. Interfaces, 2018, vol. 10, pp. 43144–55.
W. Zhang, X. Jia, Y. Li, and C. Fang: J. Appl. Phys., 2014, vol. 115, p. 17A768.
D. Liang, J.-C. Tseng, X. Liu, Y. Cai, G. Xu, and J. Shen: Materials, 2021, vol. 14, p. 929.
J. Gu, M. Song, S. Ni, S. Guo, and Y. He: Mater. Des., 2013, vol. 47, pp. 706–10.
J. Fornell, S. González, E. Rossinyol, S. Suriñach, M.D. Baró, D.V. Louzguine-Luzgin, J.H. Perepezko, J. Sort, and A. Inoue: Acta Mater., 2010, vol. 58, pp. 6256–66.
M. Pacella, V. Nekouie, and A. Badiee: J. Mater. Process. Technol., 2019, vol. 266, pp. 311–28.
F. Yang, K. Geng, P. Liaw, G. Fan, and H. Choo: Acta Mater., 2007, vol. 55, pp. 321–27.
J. Fornell, A. Concustell, S. Suriñach, W.H. Li, N. Cuadrado, A. Gebert, M.D. Baró, and J. Sort: Int. J. Plast., 2009, vol. 25, pp. 1540–59.
M. Stoica, J. Eckert, S. Roth, Z.F. Zhang, L. Schultz, and W.H. Wang: Intermetallics, 2005, vol. 13, pp. 764–69.
D. Liang, X. Wei, C. Chang, J. Li, X. Wang, and J. Shen: J. Alloy Compd., 2018, vol. 731, pp. 1146–50.
H. Zhai, X. Li, W. Li, B. Cheng, D. He, X. Zhang, and S. Cui: Surf. Coat. Technol., 2021, vol. 410, p. 126962.
S. Hasani, P. Rezaei-Shahreza, A. Seifoddini, and M. Hakimi: J. Non-Cryst. Solids, 2018, vol. 497, pp. 40–47.
R.L. Narayan, P. Tandaiya, R. Narasimhan, and U. Ramamurty: Acta Mater., 2014, vol. 80, pp. 407–20.
Funding
This research was supported by NCBiR (The National Center for Research and Development) within Grant No. LIDER/32/0178/L-10/18/NCBR/2019.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Małachowska, A., Żrodowski, Ł., Morończyk, B. et al. Selective Laser Melting of Fe-Based Metallic Glasses With Different Degree of Plasticity. Metall Mater Trans A 54, 658–670 (2023). https://doi.org/10.1007/s11661-022-06913-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11661-022-06913-w