Abstract
The US Department of Energy is working with fuel vendors to develop accident tolerant fuels (ATF) for the current fleet of light water reactors (LWRs). The ATF should be more resilient to loss of coolant accident scenarios and help extending the life of the operating LWRs. One of the proposed ATF concepts is to use iron-chromium-aluminum (FeCrAl) alloys for the cladding of the fuel. A concern in using ferritic FeCrAl is that this type of cladding may result in an increase in the concentration of tritium in the coolant. The objective of the current critical review is to collect and assess information from the literature regarding diffusion or permeation of hydrogen (H) and its isotopes deuterium (D) and Tritium (T) across industrial alloys (including FeCrAl) used or intended for the nuclear industry. Over a hundred years of data reviewed shows that the solubility of hydrogen in ferritic alloys is lower than in austenitic alloys but hydrogen permeates faster through a ferritic material than through austenitic materials. The tritium permeation rates in FeCrAl alloys are between those in austenitic stainless steels and in ferritic FeCr steels. The activation energy for hydrogen permeation is approximately 30 pct higher in the austenitic alloys compared with the ferritic (typically ∼ 50 kJ/mol in ferritic vs. ∼ 65 kJ/mol in the austenitic). None of the major elements in FeCrAl alloys react with hydrogen to form detrimental hydride phases. The effect of surface oxides on FeCrAl delaying hydrogen entrance into FeCrAl alloy is not part of this review.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Monolithic FeCrAl alloys are candidate materials to fabricate the cladding tube for uranium dioxide (Urania) fuel pellets in accident tolerant fuels (ATF) to replace the currently used zirconium (Zr) alloys.[1] The concept of ATF was born after the tsunami of March 2011 caused severe damage to several nuclear power stations (NPS) at the Fukushima Daiichi site. The common-cause failure of at least four NPS in Fukushima resulted in station blackout impairing the emergency core cooling after the safe shutdown of the reactors following the Tohoku earthquake of magnitude nine. The lack of cooling resulted in the rapid rise in temperatures of each reactor core which included approximately 80,000 fuel rods encased in Zircaloy cladding with total mass of Zr of up to 40 tons in each core.[2] The Zr metal reacted vigorously with the high temperature water and steam—the reaction itself being that of exothermic oxidation—producing large amounts of heat, zirconium dioxide, and combustible hydrogen gas that eventually escaped the reactor core and accumulated in the external building where it explosively ignited triggering a global anxiety regarding the safe use of civilian nuclear power.
The events at the Fukushima site motivated a worldwide re-evaluation of the materials used to build NPS for over six decades.[1] There was an international consensus on the need to remove all the zirconium (Zr) based materials from the light water reactors (LWR) and replace them with ATF materials also known as advanced technology fuels (ATF).[1,2,3,4,5,6,7,8,9] The newer ATF materials may include both cladding materials and fuel materials.[1] For the cladding applications there are three main concepts accepted by the thirty countries which are currently using LWR for the generation of electricity. These concepts include (in order of fastest practical implementation): (1) coated zirconium alloys, (2) monolithic iron-chromium-aluminum (FeCrAl) alloys, and (3) silicon carbide-based materials.[1]
As mentioned above, one of the ATF concepts proposes the use of FeCrAl alloys for cladding of the uranium dioxide (UO2) fuel,[10,11] instead of the current Zircaloy claddings being in use for over 60 years. The FeCrAl alloys are a modified form of (ferritic) stainless steels, in contrast to the more commonly known/used austenitic type FeCrNi stainless steels. The primary focus of this review is on the permeation characteristics of tritium through structural materials including the FeCrAl alloy system and for fuel cladding under LWR conditions. Some of the aspects discussed include (1) Hydrogen diffusion with consideration of isotope effects (relating to tritium), (2) Effect of ferritic vs austenitic lattice and effect of alloying elements in FeCrAl steels, (3) Effect of temperature up to beyond the normal LWR service (but below accident type conditions), (4) Hydrogen partial pressure effect on uptake and permeation, etc. Another important variable such as the effect of surface oxides (e.g., chromia and/or alumina) on hydrogen uptake by FeCrAl alloys is too extensive and therefore will be reported separately in another review by the same authors.
1.1 What are FeCrAl Alloys?
The initial development and patent for FeCrAl alloys dates back to 1926–1929[12] in support of high fire-resistant and high electrical-resistant materials at temperatures even above 1273 K, for applications such as furnace components and heater elements[13,14] with better properties, workability, and economics compared with nicromes then in use. Additional development led to the use of FeCrAl in other commercial applications such as the automotive catalytic converters requiring superior oxidation resistance at high temperatures as well. Although this original FeCrAl alloy development was prior to the advent of nuclear reactor systems, the beneficial characteristics of FeCrAl made them desirable for nuclear applications mainly due to their ferritic nature,[15] their excellent mechanical properties and their unparalleled resistance to oxidation at 1000 °C or higher temperatures.[15,16,17,18,19]
Within three decades of their discovery (to about 1960), the FeCrAl alloy system was well investigated with application limits well established for phase stability, workability, oxidation/corrosion resistance, and commercial viability.[20,21] A detailed investigation of the physical metallurgy of iron alloys containing 25 to 35 wt pct chromium and 3 to 8 wt pct aluminum, defining their limits for structural applications, was already well known prior to 1960.[22] Including limits of the sigma- and alpha- phase formation/solubility, the FeCrAl ternary phase diagrams from room temperature to 1173 K were presented[22] complementing Kornilov’s earlier work.[23,24] The superior high temperature oxidation resistance (up to 1573 K) of FeCrAl, was attributed to the dense protective surface film of nearly pure alumina, suggesting their use for boiler tubes as well.
For medium to high temperature reactor applications up to 1089 K, several FeCrAl alloys (23–27 wt pct Cr and 3–6 wt pct Al), a Fe–Cr–Al–Y alloy, as well as a commercial FeCrAl alloy (AISI 406 stainless steel, commonly used for electrical resistance heating element) were investigated and found to be attractive both from oxidation and corrosion resistance considerations.[19] Pessl considered the commercial AISI 406 steel to be most promising as an economic fuel cladding and a reactor core component material.[19] Also, the corrosion tests included LWR type environments (pH 10 to 7 at 563 K to 733 K and 10.3 to 22.1 MPa pressure, deionized water) and showed similar or better resistance to intergranular and stress corrosion cracking compared to the standard austenitic stainless steels,[19] and resistant in high temperature steam environment as well,[25,26] in support of the fuel cladding FeCrAl applications in LWRs.[15]
In a detailed study of gas-cooled reactor designs for mobile applications[27] the use of Fe–25Cr–4Al was specified in one design as the cladding material with yttrium-hydride moderated fuel pins on the inside and coolant gas (air) on the outside of the cladding tubes, 0.25 mm wall thickness, under relatively high average nominal cladding temperature of about 1000 K in operation; a very thin layer of oxide facing the coolant air was also expected to provide hydrogen retention during the operation. Another early work on cladding application also discussed the development of a reactive element (yttrium)-added alloy (Fe–25Cr–4Al–1Y) that was suggested for its low-stress application of the fuel cladding tubes.[28] GE studies from 1960s[29,30,31] also characterized the physicochemical and metallurgical behavior of these FeCrAl alloys for nuclear material applications. The FeCrAl alloys were first produced in the traditional melting, casting and forging method but eventually powder metallurgy was used to manufacture some versions of FeCrAl alloys. Powder metallurgy processing was first suggested in the mid-1960s to obtain better mechanical properties and specially to avoid the low elongation to failure at near ambient temperature of the cast products.[32,33] The use of powder metallurgy allowed for the alloy to have not only exceptional oxidation resistance plus high temperature strength and creep resistance but also much-needed manufacturability at near ambient temperature.[34] The increase in mechanical properties of the powder produced FeCrAl materials was mainly a result of the smaller grain sizes and the presence of dispersed oxides which are obstacles for dislocation motion. From these early studies of FeCrAl it is also apparent that one extra advantage or attractive benefit considered was the commercial availability of the alloys and the use of simple and abundant inexpensive (non-strategic) alloying elements such as Fe, Cr and Al.
1.2 FeCrAl Alloys for Nuclear Fuel Cladding in LWRs
Prior to 1982, 12 boiling-water reactors (BWRs) and nine pressurized-water reactors (PWRs) were reported to have employed stainless steel cladding for the fuel, while 1 BWR and 4 PWR units commercially in operation since mid-1960s were reportedly still using steels in 1982.[35] The earlier service performance of stainless steel cladding used in LWRs, prior to switching to the use of Zircaloy cladding, was reviewed and assessed in comparison with that of the Zircaloy in the same EPRI report.[35] The switch from stainless to Zircaloy was mainly for economic reasons i.e., reduced fuel cycle costs attributed to better neutronics of the Zircaloy cladding. During the time of the materials switch the production of Zircaloy was less expensive so it could be used for civilian power applications. The austenitic FeCrNi steels were mainly of the series 300, primarily type 304 stainless steel. The performance of the steel materials was reviewed and assessed with respect to the fuel integrity under various likely accident conditions at high temperature, and related oxidation, margins to their cladding failure, and hydrogen release. From this assessment Strasser et al. observed that (a) below the peak clad temperature (PCT) of 1089 K there was little reason to prefer Zircaloy over the austenitic FeCrNi stainless steels, (b) for a PCT between 1089 K and 1477 K, the stainless steels would have better performance than Zircaloy, and (c) for severe accident conditions (PCT reaching above 1477 K) the FeCrNi stainless steels did not seem to offer advantage over Zircaloy cladding.[35] However, it is to be noted that under the severe but rare accident conditions, with PCT exceeding 1477 K, zirconium alloy cladding system has been now deemed susceptible to severe degradation.[10]
Also prior to the Fukushima 2011 event, alternate materials and coatings for the Zircaloy cladding in LWRs were examined specifically to enhance the system safety under severe accident conditions so as to increase the time to cladding failure and to reduce the hydrogen production that would otherwise increase the risk of containment explosion.[36] For example, alloys based on molybdenum (“TZM”) or niobium (“B-66”), with increased strength and oxidation resistance at higher temperatures, and silicide or aluminide coatings were identified candidates; that work, as well as the above review by Strasser et al.,[35] did not include or discuss the use of FeCrAl alloys.
Prior to and since the above early reviews on cladding in LWR service, it has been commonly observed that a cladding material of preference should, as a minimum, offer high oxidation/corrosion resistance in conjunction with high strength, both at high temperatures, and good neutronics properties (such as a low thermal neutron absorption cross-section)—in addition to proven corrosion resistance and acceptability for long-term operation (relative to the fuel cycle) under normal LWR service conditions. After completing the LWR service (fuel-cycle) the fuel bundle with cladding also needs to maintain integrity and radioactive safety levels during the shorter-term (on-site fuel pool) and longer-term (dry-casks) storage.
The FeCrAl alloys have excellent resistance to aqueous corrosion,[15,37,38,39,40] superior resistance to oxidation in high temperature air[17,20,41] and steam,[10,40,42,43,44,45] and better creep/mechanical properties expected at high temperatures.[10,46,47] The superior high-temperature oxidation resistance of FeCrAl reduces the generation of hydrogen and associated exothermic heat of reaction with coolant during severe accidents,[45,48,49] while the creep/mechanical properties enhance the cladding burst margin.[46,47] FeCrAl alloys (and especially APMT) have an outstanding environmental resistance under the normal LWR coolant conditions, in addition to their excellent oxidation resistance under potential severe accident conditions. The good corrosion resistance has been attributed to high Cr concentration (leading to chromium oxide film) and the high temperature oxidation resistance is due to the formation of an aluminum-oxide (α-alumina) surface layer.[8,40,44]
2 Objectives of this Review
2.1 FeCrAl and Hydrogen
In general, due to the ferritic (α-iron type, bcc) structure, the FeCrAl alloys are expected to have greater diffusivity and permeability to hydrogen and hence to its radioactive isotope, tritium, in comparison with the austenitic stainless steels and zirconium alloys of the current or former claddings. At the same time, due to their greater absorption cross-section for thermal neutrons, the cladding of FeCrAl is expected to be thinner compared to Zircaloy.[50,51] As such, these distinguishing aspects of tritium diffusion through the cladding under LWR operating conditions need to be evaluated and assessed for the FeCrAl alloys.
Strasser et al (1982) also reviewed the comparative tritium release aspects of the (austenitic) stainless steel and Zircaloy claddings for LWRs, where the primary source of tritium was known to be the ternary fission product in UO2 fuel pellets and the resulting tritium gas would be migrating through/out of the pellets into the fuel–clad gap, entering the clad inside surface, diffusing through the cladding wall and its protective oxides, exiting into the primary water coolant.[35] Eventually the tritium may escape to the environment via released steam. This process of tritium permeation in general and of its diffusion through the candidate FeCrAl fuel cladding in particular are of interest to this review. Additionally, from the point of view of resource utilization and likely demand for longer fuel cycle there is greater incentive to better address the issues related to tritium permeation while planning for and implementing the newer fuel technologies.
In the case of LWRs, the cladding inside surface has the fuel-burnup induced tritium containing dry gaseous environment with very low oxygen potential, while the outside surface has the typical aqueous environment of high purity oxygenated water in BWRs or lithium-borated, hydrogenated water in PWRs (Figure 1).[8] In BWRs, the tritium is generated primarily from ternary fission, burnable poisons, and deuterium activation; also, due to the absence of boric acid in the primary coolant and lower operating temperatures in a BWR, relative to a PWR, the tritium diffusion (and its accumulation in the reactor coolant system) is lower in the BWR systems.[52,53,54] The cladding temperatures for typical service conditions in PWRs and BWRs were reported to be 630 ± 5 K and 590 ± 5 K, respectively.[55]
Representation of the FeCrAl cladding condition under normal operation conditions in a light water reactor. The FeCrAl wall will develop an alumina oxide on the cavity side and a chromia oxide on the water side. Both oxides are barriers for tritium diffusion from the fuel cavity into the coolant. Not to scale
Because of the significantly large production and inventory of tritium in fusion systems—compared to the fission based LWR energy systems—considerable effort in the fusion related structural materials development deals also with their hydrogen/tritium permeation. Only a selection of literature from this vast field is reviewed here for illustrating the principles and some data for reference in relation to the LWR focus of this survey. Excluded from this review are considerations of special surface treatments and coatings on structural materials which are likely to be used as tritium permeation barrier in some cases.[56,57,58,59,60,61,62]
2.2 Tritium in Reactors: Implications of Using FeCrAl Alloys
The behavior and properties of tritium transport have been of interest and, as such, have been investigated for safety and maintainability purposes in various reactor systems since early 1950s. These systems included: the fission type BWR,[53] PWR,[52] liquid metal fast breeder reactor (LMFBR),[29,63] high temperature gas-cooled reactor (HTGR), heavy water reactor (HWR), molten salt breeder reactor (MSBR),[64,65] high temperature reactor (HTR),[66] and more recent international designs of the fusion type systems, e.g., International Thermonuclear Experimental Reactor (ITER) and DEMOnstration Power Plant (DEMO).[67,68]
The diffusion and permeability of tritium/hydrogen in these nuclear systems dealt with:
-
(a)
Various major components including fuel claddings, heat exchanger tubes, fusion breeding blankets,[68] coolant piping, and tokomak fusion test reactor (TFTR) vacuum vessels.[69]
-
(b)
Various classes of structural materials: Zircaloys, variety of steels,[16] austenitic stainless steels, reduced activation ferritic-martensitic (RAFM) steels,[70,71] Fe–Cr ferritic steels, and Fe–Cr–Al ferritic steels.[19,27,28,29]
Results on hydrogen permeability of similar FeCrAl alloys were presented in some early publications[72,73] details of which are discussed later below. Several comprehensive reviews relating to the permeation of tritium/hydrogen in the above-mentioned materials–systems have been published over the years;[2,16,37,74,75,76,77,78,79] these reviews are included by reference in the subsequent sections.
Generally speaking, the focus of tritium permeation work in more recent (fusion) systems was on the structural materials of the FeCr variety. As such, the earlier interest in more specific FeCrAl (ternary) alloys remained relatively limited until the potential benefits of these were re-identified in their application for ATF claddings. Nevertheless, the very early works in various reactor systems clearly included many investigations into the FeCrAl alloy family which are relevant. While the work on FeCr variety of alloys is also useful to consider because of the similarities in the crystal structure and related thermo-physical properties of the two classes of ferritic alloys. Both these works are used in this review. Compositions of typical alloys of interest or those referred to in this review are listed in Table I.
3 Hydrogen (H, D, and T) Permeation: Review and Assessment
Tritium is one of the three stable isotopes of hydrogen. Tritium is the third isotope of hydrogenFootnote 1—with two additional neutrons in its nucleus—that is, Tritium is radioactive, with a half-life of 4500 days,[80] decaying by the emission of very low energy beta radiation. As a consequence, the gas form of tritium (T2) is also radioactive, and as an isotope, it is chemically and physically similar in response to that of the gaseous hydrogen (H2). As such, permeation of tritium can be viewed and considered in conjunction with and similar to that of hydrogen and deuterium (D), with an adjustment factor for the isotopic mass difference effect discussed in a subsequent section.
3.1 The Diffusion Process and the Controlling Parameters
The hydrogen response, such as its absorption, accumulation, interactions, and leakage, to/from/within structural materials is primarily determined by the transport properties of hydrogen in a given material. That is, the hydrogen isotope permeation process and its rate are typically described in terms of the parameters called the permeability, diffusivity,Footnote 2 and solubility which are defined below. In the current context, all three parameters are defined for or within a solid substance, namely, in metal alloys and their oxides.
Solubility refers to the ability of a solid to solubilize or dissolve H, D, or T, and its quantification. Diffusion refers to the process of migration of H, D, or T within the solid and diffusivity refers to its quantification. Permeation, on the other hand, refers to the overall process of transfer and release of hydrogen (isotopes) from one side/surface of a component/system to the other, and permeability refers to quantification of the permeation. As such, permeation is more complex, involving a diverse set of connected and consecutive processes, e.g., dissociation/recombination of hydrogen (gas) molecules at the barrier (cladding) surface, adsorption/desorption at or on the surfaces, dissolution/trapping in the oxide layer(s) and the diffusion (with or without chemical reactions) within the oxide and in the bulk of metallic thicknesses. That is, the permeation process and its parameters incorporate and are subject to the characteristics of these additional processes prevailing, including those of the surface and environment conditions on both sides, in addition to the primary absorption and diffusion through the solid.
Both the diffusion and permeation are also affected by the solubility of hydrogen and its interaction (trapping, hydriding, etc.Footnote 3) within the solid. Since the various processes are consecutive, the overall permeation kinetics is typically governed by the slowest step process. The empirical assessment of permeation, however, can be somewhat simplified especially under steady state condition as is the focus in the following treatment. Under most situations, it is often found or assumed that the permeability is related to the product of solubility and diffusivity[74,77,81,82]—when diffusion through the solid material is the rate controlling process and surface reactions have negligible influence on the overall permeation. Also, when the permeation is diffusion-controlled, the near-surface concentration of hydrogen under equilibrium is governed by the Sieverts’s law.[83] This concentration is primarily determined by the hydrogen partial pressure and temperature as discussed below.
The following set of simplified equations is given, to define the quantities of interest and to enable discussion of the permeation related data reviewed here, mainly from the empirical point of view, although there is some theoretical basis for these equations for which the original or other references cited below may be consulted.
3.2 Diffusivity of Hydrogen and Isotopes Across the Cladding Wall
Independently of any surface effects/reactions the actual movement of hydrogen, from one side of a boundary wall to the other (in the current context, from the higher pressure fuel cavity of the cladding tubes to the coolant side) occurs by diffusion. This diffusion of hydrogen across the cladding wall is governed by the simple and well accepted expression attributed to Fick.[77,84] Fick’s first law of diffusion states that the steady state flux, F, (i.e., the net quantity of diffusant crossing normal to a section of unit area per unit of time) is proportional to the diffusant’s concentration gradient down the normal direction.[85,86] That is, for the case of one-dimensional diffusion normal to the surface/section:
where, F is the flux (mol/m2∙s), D is the diffusivityFootnote 4 of the diffusant (hydrogen) in the host (FeCrAl) (m2/s), C is the concentration of the diffusant in the host (mol/m3), and x is the position coordinate along the direction of diffusion (m) or across the wall thickness of the cladding.
Thus, diffusivity represents the quantity of diffusant passing through a unit area per unit time across the unit gradient of its concentration.Footnote 5 In the current context: (a) the one-dimensional form is adequate for a thin tube, and (b) for the case of materials with bcc or fcc structures the cubic symmetry implies the adequacy of using D as a scalar isotropic parameter for the flux. Note that when the amount or quantity of diffusant in C and F is measured in any same unit, here it is in moles, the unit of diffusivity (with dimensions of L2t−1) is not affected. The parameter D applies to or characterizes a given pair of diffusant specie and host material; it is a fairly strong function of temperature and, in general, it may depend on the concentration. The temperature dependence follows the well-known Arrhenius relation over a broad range as reported below and in a separate section.
3.3 Solubility of Hydrogen in the Cladding Wall
For the case of an ideal diatomic mononuclear gas dissolving to form an infinitely dilute solution in the solid, the solubility can be given by the Sieverts’s law[83] that relates the specie’s gas (i.e., hydrogen) pressure to its atomic concentration in thermodynamic equilibriumFootnote 6 just below the interface:
where, S is the solubility (mol/[m3∙√MPa]), p is the species gas (partial) pressure at the interface (MPa).
The proportionality constant, S, is related to the equilibrium constant for the reaction (1/2)∙H2(gas) ↔ H(s) at temperature and it is also called the Sieverts’s constant for the dissolution of gas into a solid. Note that the units of solubility have associated with it the pressure unit and it differs from the usual notion of concentration (such as expressed in percent or ppm). From the above two equations it may be noted that both the concentration of dissolved hydrogen and diffusion flux are influenced by the equilibrium gas pressure at the solid–gas interface. In 1937 Fowler and Smithells[87] have provided some theoretical considerations leading to the square-root dependence of hydrogen solubilityFootnote 7 in metals and supporting its atomic absorption. However, deviations from the square-root pressure dependence in the Sieverts’s law have been noted in the past at extremes of the partial pressure, particularly at very low end well below 1 × 10−7 MPa, which are discussed separately later.
3.4 Permeability of Hydrogen Across the Cladding Wall
Note (from the Fick’s law, Eq. [1]) that the mass flux of tritium diffusion is dependent on the concentration gradient which—for a thin layer or component/specimen wall, with small ratio of the thickness, δ, to diffusion cross-section area, A—is uniform/linear and inversely proportional to the thickness. In this case, combining or integrating the above relations for diffusivity (Eq. [1]) and solubility (Eq. [2]), with boundary conditions at the two interfaces of a thin layer/wall maintained at respective partial pressures of p1 and p2, gives the following expression for the steady-state diffusion flux:
Where, c, s, and p denote the concentration, solubility, and pressure at an interface, subscripts 1 and 2 refer to the two sides/interfaces with high and low pressures (fuel cavity and coolant side, respectively), and n = 1/2 for the Sieverts’s law. Assuming the p2 term (also identified as downstream pressure) to be negligible compared with the p1 term (upstream or driving pressure), with p1 = p and n = 1/2, the steady state rate of permeation (mass flow per unit time) normal to the cross-section is given by the following expression:
Where, Φ is the rate of hydrogen or tritium permeation (mol/s), A is the area of permeation (m2), δ is the thickness or interfacial distance (m).
The Eq. [4] expression gives the permeation rate in terms of the diffusivity and solubility of the solid–diffusant (FeCrAl–Tritium) pair, the external driving partial pressure, and the geometric parameter. Both (S and D) affect the net flow (permeation) of the diffusant from one side of a component or specimen to the other side. In principle, to describe the permeation response, the combination of D and S as measured or estimated should come from the same source. The form of expression, Eq. [4], for permeation rate of a gas through metal and its dependence on (gas) pressure and temperature have been generally attributed to the 1904 work of Richardson et al.[88] who supported it with theoretical considerations in agreement with their experimental data on hydrogen through a thin platinum tube. The work by Richardson et al.[88] is briefly revisited below under the section on pressure and temperature dependence of (H, D, T) permeation.
The product of diffusivity (D) and solubility (S) as the proportionality term in the above expression is defined as the permeability (P) characterizing the solid–diffusant pair at a given temperature in a given external environment. That is, permeability is the rate of flow of a diffusant crossing unit area and through unit thickness of a fixed layer of solid, per unit partial pressure on one interface and nearly zero partial pressure of the diffusing species at the other interface as noted above. Thus, the above expression for the rate of permeation can be written as:
Where, P = D × S is the permeability (mol/[m s √MPa]).
The above defined parameters (D, S, and P) quantifying the permeation rate or flux are dependent on the temperature. This dependence follows the exponential form, well known as the Arrhenius relation, particularly for permeation of a gas through metallic solids, which has been well supported by experimental works over a wide range of temperatures and materials.[74,82,84,89] The thermodynamic and statistical-mechanical underpinning of the diffusion and solubility processes also provide some theoretical support for the exponential temperature dependence. This is expressed by the following relations:
Where, D0 is diffusivity constant or diffusivity pre-exponent (m2/s), S0 is solubility constant or solubility pre-exponent (mol/[m3 √MPa]), P0 is permeability constant or permeability pre-exponent (mol/[m s √MPa]), Qd is activation energy of the diffusion process (kJ/mol), Qs is heat of absorption in the solid (kJ/mol), Qp is activation energy of the permeation process (kJ/mol), T is temperature on the absolute scale (K), R is the universal gas constant (8.3145 × 10−3 kJ/mol K).
The constants D0, S0, and P0 for a given pair of difussant–solid (Tritium–FeCrAl) also represent the limiting values of diffusivity, solubility, and permeability, respectively, at the extremely high (infinite) temperature. In addition, from the above relation of P = D × S and Eq. [6] it follows that: P0 = D0 × S0 and Qp = Qd + Qs, where, in principle, the diffusivity and solubility parameters are obtained from the observations and tests made with a given experimental setup and conditions.
3.5 Related Notes
Relative to other chemical elements the diffusion rate of hydrogen is generally the fastest for given conditions. The process of diffusion and resulting permeation are affected by the mass of the diffusant, the lower the mass the higher is the rate of diffusion and related permeation, while the solubility is relatively unaffected by the mass. Tritium being the heaviest stable isotope is expected to have lower diffusivity and permeability compared to hydrogen, other conditions remaining the same. This isotopic effect and related adjustment for the above D and P parameters are discussed in a subsequent section.
The diffusion of hydrogen within a base alloy or metallic substrata, generally in atomic form, typically involves interstitial migration through the (poly) crystalline structure, with some grain-boundary diffusion depending on the temperature. Hydrogen trapping within the substrata, more so at lower temperatures, reduces the availability of diffusible hydrogen thereby decreasing the net permeability. In addition, the concentration gradient of hydrogen through the bulk alloy region is reduced by intervening oxide (or a barrier surface coating), thereby reducing the rate and total permeation (of H, D, or T). Oxide layers (native or coated) impede the permeation, typically by orders of magnitude, and this reduction effect is reviewed in another article. Furthermore, with increasing temperature the steady state permeation rate is expected to be reached relatively quickly and this rate becomes more sensitive to temperature within the similar class of materials and oxides as compared with their transient permeation response. These effects and observations are generally applicable. Therefore, it is of interest to examine the permeation parameters of interest within a wide range of similarly structured or a class of alloys and/or similar oxide types/formations. This broad classification or generalized treatment is also commensurate with the observation of a fairly wide scatter and uncertainty with respect to the experimental data and estimation methods used in determining the diffusion/permeation parameters.
4 Permeation of Hydrogen Isotopes in FeCrAl Alloys
One of the early studies of hydrogen permeation rate response of a FeCrAl alloy (nominally: 20 pct Cr, 5 pct Al, and balance Fe) and (ferritic) AISI Type 446 stainless steel (nominally: 25 pct Cr and balance Fe) (Table I), with and without the oxide layer, was presented by Huffin and Williams.[90] Although this work was carried out at relatively high temperatures (above 922 K up to about 1450 K), the presence of a continuous surface oxide film resulted in nearly three orders of magnitude reduction in the steady-state permeation rate compared with that of the unoxidized (clean) surface alloy. More details on surface effect on hydrogen permeation are discussed in a separate article.
In connection with the use of iron-based (ferritic-martensitic) steels in fusion reactor applications and related implications for tritium migration, Van Deventer and Maroni[91,92] reported several data and observations on the permeation of hydrogen on various steels including some FeCrAl alloys, covering the low temperature region down to 423 K and driving hydrogen partial pressures as low as 2 × 10−6 MPa. Apparently, from the context in the related discussions, the Van Deventer data for FeCrAl alloys were on samples oxidized as-received or during testing; that is, the compiled data from their work displayed as general bands for three nominal FeCrAl compositions did not include specific or intentional oxidation treatment. For discussion, the data are best summarized here as the respective central (mean) Arrhenius lines for tritium permeation between ~ 250 °C and 1000 °C (Figure 2).Footnote 8 Also shown in Figure 2 are representative tritium permeation lines for several materials of interest including bcc α-iron and a few other steelsFootnote 9 from other reports for comparison (Table I). Figure 2 also includes the above noted data on the FeCrAl alloy and Type 446 ferritic steel in the higher temperature region.[90]
Figure 3 shows tritium permeation values of mostly FeCrAl in clean surface conditions.
The maximum predicted temperature of a prospective FeCrAl cladding would be in the order of 400°C (673K). According to Figures 2 and 3, the tritium permeation at ~ 400 °C would be the highest for alpha iron (4.5 × 10−8), followed by type 406 (13Cr) steel (7.3 × 10−9) and the lowest is for APMT (1.5 × 10−9), in units of Figures 2 and 3. All these materials are ferritic, but they contain an increasingly higher amount of alloying elements, which seem to show negative effect on the rate of tritium permeation.
Based on their review of past work in 1984, Swansiger et al.[94] reported that the use of iron-chromium alloys containing aluminum, with oxidized surfaces, for tritium permeation barrier was a possible better alternative to other iron-chromium alloys or aluminum coated alloys. That publication was focused on the role of oxidized surface conditions of a Fecralloy (with 15.8 pct Cr, 4.8 pct Al, 0.3 pct Y) on deuterium permeation at low (below 723 K) temperatures; as such, these results are discussed more fully in a separate review. Swansiger et al.[94]did include for reference some data on the permeation response of the alloy in clean non-oxidized (sputter-cleaned palladium coated) condition which may be considered as an upper estimate of the hydrogen isotope permeation in similar Fecralloy materials; the permeation parameters for this clean condition are included in Table II and in subsequent comparisons.
Measurements on the hydrogen permeability of three FeCrAl alloys (one commercial grade FeCrAl alloy: APMT, and two specially prepared wrought nuclear grade FeCrAl alloys: T35Y2 and T54Y2), in the mid-range of temperature, between 623 K and 923 K, were reported in a study by Hu et al.[78]. Note that these measurements were performed with a static pressure change method—much different from the commonly used gas evolution permeation technique—also, the Hu et al. method utilized a feeding tube requiring a correction for its hydrogen leakage. This should be considered in comparing or applying these permeability data further. Hu et al.[78] study did include some limited calibration and validation of the measurement system with permeability tests using a Type 304 stainless steel and comparing the results with data from selected prior works. The researchers considered their comparison to show adequate agreement, but with somewhat lower permeability (note: for their Type 304 stainless steel data the estimated activation energy of about 75 kJ/mol is somewhat on the higher end). Nevertheless, their results on the three FeCrAl alloys are included in Figure 3, with additional comparisons and discussion in the following sections.
Sakamoto et al.[6,95] have performed several investigations on the performance and application of oxide-dispersion-strengthened (ODS) type FeCrAl alloy (Table I) to BWR fuel claddings, which included data and assessment of their corrosion response (in BWR type water) and its influence on the tritium permeability below 573 K. The material composition was reported to be Fe plus 11.6 Cr, 6.15 Al, 0.49 Ti, 0.39 Zr and 0.48 Y2O3 all as wt pct.[6] The permeation response was measured prior to corrosion (oxidation) as well as after the corrosion in an autoclave with circulating water containing 8 ppm dissolved oxygen and at 563 K for 30 days. The corrosion-formed oxide layer was mechanically removed from one side prior to the permeation measurement. Permeation data on these specimens were reported at steady state, with decreasing temperatures to 423 K (for corroded samples) and to 343 K (for non-corroded samples). From these data the permeation parameters for the two types of samples are estimated here and given in Figure 3 comparisons: in each case the activation energy is estimated to be about 43 kJ/mol, and the ratio of pre-exponents is estimated to be about 3.3. This permeability reduction factor (for the corroded samples) was viewed to be less than expected, leading to additional tests on the corroded samples measuring the permeability at stepwise 50 K increase in temperature, from room temperature to 573 K, with hold of 1 hour at each step. No measurable permeation was found in these non-steady state steps to 573 K, and the permeation increased only beyond 2 hour holding period at 573 K.
Similarly, measurements of tritium permeability in FeCrAl ferritic steel were reported in the temperature range of 373 K to 623 K, both for oxidized surface condition and non-oxidized condition.[96] Although the steel was of the ODS variety containing Ce oxides, the permeability for non-oxidized material was found to be consistent with that of other common ferritic steels and FeCrAl steel as discussed below. The above results on ODS FeCrAl steels are also shown in Figure 3. As expected, the permeability was reduced by the oxide layer (this is discussed further in a separate article).
5 Observations on Hydrogen Isotope Diffusion in FeCrAl
Figure 2 shows a broader comparison of tritium permeability response of FeCr steel alloys with or without aluminum, mostly in as-received oxidized or non-oxidized conditions and includes α-iron (bcc) response for baseline reference. Figure 3 shows a similar but more specific comparison of permeabilities in the case of representative FeCrAl alloys, mostly in clean or non-oxidized conditions.
Generally, and as expected, Figure 2 shows that the permeability range in specifically oxidized condition is significantly lower compared to that of the clean/non-oxidized materials. Figures 3 and 4 show that the permeation of tritium through FeCrAl seems to have a similar activation energy to that of the permeation of tritium through FeCr alloys (e.g., 406, 410 and 420 steels). According to Hu et al.,[78] the activation energies for the APMT and T54Y2 seemed slightly higher (Reasons for this exception are not apparent). The ODS variety of FeCrAl alloys in the clean/non-corroded condition (Figure 3) show moderately higher tritium permeability response relative to the same reference line for of Type 406 steel shown for comparison. For clarity, a reduced set of these permeability responses are re-plotted as shown in Figure 4, excluding the noted APMT and T54Y2 exceptions, which support the noted observations.
Based on the above data assessment and the results shown in Figure 4 the following bounds and central trend are estimated as reasonably conservative for the clean/unoxidized condition of the FeCrAl alloys, including the ODS variety:
-
Upper bound (estimate): P0 = 7.84 × 10−5 mol/[m s √MPa] and Qp = 48.0 kJ/mol
-
Central trend (estimate): P0 = 4.90 × 10−5 mol/[m s √MPa] and Qp = 49.5 kJ/mol
-
Lower bound (estimate): P0 = 3.06 × 10−5 mol/[m s √MPa] and Qp = 51.0 kJ/mol
These are empirical and approximate but acceptable for further comparisons and discussion below, given the expected data uncertainty. These are somewhat wider as they include/address the ODS data.
6 Permeation of Hydrogen in Related Steels
Permeation rates of hydrogen in ferritic steels are generally higher than those in austenitic steels; this general observation is related partly to the lower activation energy of hydrogen diffusion expected in the more open bcc structure of ferritic steels compared to the more densely populated fcc structure of austenitic steels.Footnote 10
It is instructive to note some observations made by Bell et al.[93] who compared permeabilities of seven representative structural metallic materials that included ferritic, austenitic, and high nickel alloys (in clean or nonoxidized condition); they suggested that:
-
(a)
the range of permeability values may well represent most nickel and iron based alloys,
-
(b)
the alloys can be separated into two groups for tritium permeability—ferritic alloys with activation energy of about 50.21 ± 3.35 kJ/mol, and austenitic alloys with 66.94 ± 0.84 kJ/mol,
-
(c)
alloy permeabilities were not significantly affected by the alloy compositions within a given group of alloys, and
-
(d)
any difference in permeabilities from or within alloy groups tended to become smaller with increasing temperatures.
Bell et al. also noted that the more open (bcc) crystal structure of ferritic materials (with lower density or greater void fraction), compared with that (fcc) of the austenitic materials, likely is the significant factor influencing the greater permeability in bcc; and, since the hydrogen solubility is inversely correlated with the structure (i.e., greater in fcc), the diffusivity in open structure ferritic materials is expected to be higher by even greater multiple than the permeability.[93] Similar observations have been made by others and are generally in agreement with the present assessment. As such, on the basis of commonality and significant influence of the crystal structure, it is instructive to review and compare the hydrogen isotope response in other related steels briefly with illustrative cases as discussed below.
Additionally, the formation of oxides (mainly Cr2O3, but also multi-layer iron-chromium spinels) on the FeCrAl alloys is similar to that on the ferrous alloys (type 300 series and nickel base alloys) commonly employed in the LWR aqueous service. That is, at normal operation conditions, both of these classes of alloys (austenitic and ferritic) offer the same passivity/protection based on the oxides under aqueous conditions. As such, on the basis of commonality and significance of oxides as well, data on hydrogen diffusion/permeation in the other related steels are of interest since oxides play a decisive role in the hydrogen transport, notwithstanding effects of oxide surface/defects and hydrogen trapping characteristics.
The hydrogen permeation in (fcc) austenitic steels as well as in (bcc) ferritic steels, in general, has been investigated extensively,[16,98] as compared to the FeCrAl alloys which are ferritic and include aluminum as an important alloying element. Of these investigations on the other steels only a small yet pertinent set of randomly selected, representative data is discussed below, in three parts, for illustration and comparative assessment in relation to the permeation response of FeCrAl alloys.
7 Permeation of Hydrogen in Austenitic Stainless Steels
The work reported by Flint in 1951 on hydrogen permeation/diffusion in materials of construction is not only one of the earliest but also very comprehensive in its scope and description; as such, results from this work are summarized only briefly in multiple sections of this review.[16] Data on several 300-series austenitic stainless steels, especially Type 347 steel, were presented. Pertinent results on permeation of hydrogen in these alloys are included in the summary Table III.
Swansiger et al. reported results of permeation on three different stainless steels (309S, A-286 and 21-6-9), with sputter-cleaned, palladium coating, in the temperature ranges of 475 K to 700 K for deuterium and 325 K to 475 K for tritium, with the fixed upstream (driving) gas pressure in the range of 0.0013 MPa to 0.1 MPa.[99] The bulk permeabilities of the three stainless steels, even with major differences in their alloy composition, were found to be nearly the same (within 30 pct of the mean), and normalization of tritium data to that for deuterium with the isotope factor of (√3/√2) matched well with the data for deuterium over the temperature range and over the permeabilities spanning five orders of magnitude. The permeability parameters from these data are given in Table III, in terms of deuterium permeabilities for the three stainless steels. Additionally, three samples of 21-6-9 stainless steel were tested with three combinations of oxidized, but palladium-coated sides for which the data showed only small variation and were reported with deuterium permeation parameters as a group. This set of parameters is also given Table III and it is close to the above response noted for the sputter-cleaned, palladium-coated bulk alloy.
Another early report on the permeation of tritium[100] dealt with the Type 304L stainless steel that has one of the lowest nickel and carbon contents amongst the common austenitic stainless steels. The measurements were carried out for permeation through the bulk steel at extremely low pressures (4 × 10−9 to 8 × 10−8 Pa)Footnote 11 between 723 K and 1023 K. The resulting estimate of permeability parameters (Qp = 73.7 kJ/mol and P0 = 3.84 × 10−4 mol/m s √MPa) are included in Table III, along with similar results from several other works. At these very low tritium pressures the authors considered their best-fit exponent of about 0.58 for pressure dependence at 961 K to be close enough to the presumed half-power dependence for the permeability response. It was noted that the activation energy of permeation was about the same for both types of stainless steels (304 and 304L), although the permeability coefficient was lower in the case of 304L likely due to its much lower carbon content.[100]
Tritium permeation tests on sputter-cleaned, Pd-coated samples of Type 304L stainless steel were reported by Maienschein et al. in the lower temperature range of 323 K to 443 K and at 0.101 MPa pressure of tritium gas.[101] The permeation parameters from this data set are estimated here and included in Table III. In addition, included in this table are data and parameters on the various austenitic steels from several other sources as listed.
Based on the review of representative data for permeation in austenitic stainless steels, which are also in good agreement with several other reports not included here, the resulting tritium permeability parameters with reasonably approximate bounds are estimated here to be:
-
Upper bound (estimate): P0 = 1.40 × 10−4 mol/[m s √MPa] and Qp = 61.5 kJ/mol
-
Central trend (estimate): P0 = 1.00 × 10−4 mol/[m s √MPa] and Qp = 63.0 kJ/mol
-
Lower bound (estimate): P0 = 7.14 × 10−5 mol/[m s √MPa] and Qp = 64.5 kJ/mol
With the exception of one set[96] showing relatively higher permeability constant but similar activation energy, and another[100] showing relatively higher activation energy, most of the data reviewed (Table III) fall within a fairly narrow band (a factor of about 1.9 on the mean). This is over a wide range of temperatures and pressures (Table III) and nearly five orders of magnitude in the tritium permeability for the annealed austenitic stainless steels. As such, within a given class of single-phase austenitic stainless steel, the influence of variations in main alloying composition and microstructure on permeation parameters is relatively small and/or within the precision of experimental results, confirming earlier similar observations [Flint 1951]. However, it has been reported that in Type 304 and Type 321 steels that are metastable where α'-martensitic (bcc) phase can be induced, e.g., by cold-working, the permeability and diffusivity increases measurably approaching that of the class of ferritic-martensitic steels, reviewed below, as the fraction of bcc phase increases.[102] Similar observations were made in an earlier work on Type 304L.[103]
8 Permeation of Hydrogen in Ferritic and Ferritic/Martensitic Steels
The permeability response of hydrogen in ferritic or ferritic-martensitic steels has also been reported by many investigators, especially since these materials were considered to be of primary interest in the more recent fusion reactor applications. Compared to the austenitic steels, significantly lower solubility but higher diffusivity along with lower activation energy of diffusion has been noted for the ferritic steels.[16,89,104] This difference was attributed primarily to the bcc versus fcc crystal structure as noted earlier (see footnote ##). Several RAFM steels—investigated for tritium containment in fusion reactor applications—also share the bcc structure and include Eurofer (European), F82H (Japanese), ARAA (Korean), and CLAM (Chinese) versions RAFM steels.
Significantly, the reduction of hydrogen permeability in ferritic steels has been noted in several studies,[72,78,90,92,105,106,107] associated with the addition of aluminum as an alloying element in these steels.
Unlike the austenitic stainless steels, especially the 300-series, the ferritic and ferritic/martensitic alloyed steels cover a much wider range of alloying additions, microstructures and/or hydrogen transport properties. After review of these properties the representative data are grouped below in two parts to facilitate the comparisons and discussion: one part includes the more common and widely investigated RAFM steels, and the other includes the 400-series steels and other developmental or specially prepared RAFM alloys.
8.1 Hydrogen in the Widely Investigated RAFM Steels
As mentioned before, RAFM are reduced activated ferritic martensitic steels. Representative data on hydrogen isotope interactions in fully martensitic steels, DIN 1.4914 or MANET reported by Forcey et al.[108] and Perujo et al.[109] are presented in Figure 5. The former used gas permeation method while the latter used gas permeation and gas evolution methods; and the resulting permeability values were closely comparable. Hydrogen permeability data on ferritic F82H steel heat-treated to fully martensitic condition reported by Serra and Benamati[110] are presented in Figure 5. The resulting estimate of tritium permeability in this fully martensitic steel is shown in Figure 5 for comparison with other ferritic steels. It shows one of the highest permeability responses among the group of RAFM type steels, consistent with the known high diffusivity of hydrogen in the martensitic phase.
The data on EUROFER, which covers the temperatures down to 376 K shows relatively low activation energy.[111] Also, the data on one ODS variety of EUROFER reported by Esteban et al.[112] shows the lowest activation energy of permeation, while still within the broader band of other RAFM steels, shown in Figure 5. They attributed this to microstructural and trapping differences primarily due to the presence of high density of yttria nanoparticles leading to low activation energy of solution and high (general) solubility in the lower temperature range, down to 420 K.
Permeation parameters for additional and other variety of RAFM steels [OPTIFER and Rusfer] show that the permeability response for this group of ferritic materials shows somewhat wide variation, as compared to that for the austenitic steels: in particular, the activation energy of permeation is generally in the range of 41 ± 4 kJ/mol, especially above about 500 K, although covering a wide variety of the RAFM type steels. The overall permeability response falls within a factor of about 1.75 on the average of the band, except for the Rusfer[113] with the highest activation energy and generally lower permeability. The resultant approximate bounding parameters are:
-
Upper bound (estimate): P0 = 3.20 × 10−5 mol/[m s √MPa] and Qp = 39.7 kJ/mol
-
Central trend (estimate): P0 = 1.83 × 10−5 mol/[m s √MPa] and Qp = 39.7 kJ/mol
-
Lower bound (estimate): P0 = 1.05 × 10−5 mol/[m s √MPa] and Qp = 39.7 kJ/mol
8.2 Hydrogen in Ferritic Type 4xx Steels and Special RAFM Alloys
Results of hydrogen permeation on Type 446 ferritic steel (with 25 pct Cr),[90] at somewhat higher temperatures, were included earlier in Tables II and Figure 2 for comparison with Fe–Cr–Al alloys. They observed significant reduction in the hydrogen permeability of Type 446 ferritic steel when aluminum content was increased even in apparently unoxidized samples. The reduction was thought to be primarily related to the presence of very thin residual oxide films that were more tenacious with (because of) the higher aluminum content. In a more recent report, Urabe et al.[96] gave permeability parameters for Type 430 and considered the response to be close enough to both their Ce-ODS material (a modified FeCrAl alloy) and to that of an earlier study on Type 430 steel at much higher temperatures [Rudd and Vetrano 1961]. Although their curve for Type 430 steel appears to be consistent, yet it is about 33 pct higher than that of Rudd and Vetrano[114] extrapolated down from the higher temperature (shown in Figure 2). Also, the data from Huang et al.[61] on Type 430 steel shows the lowest activation energy although the permeability values for the temperature range fall within the general band for non-430 materials; this may be related to the fact that only this data is based on Pd-coated, clean surface condition. However, another set of permeability data on Type 430 stainless steel[115] is significantly below all other data. Any reason(s) for these wide differences in the reported permeation responses on Type 430 steel were not immediately apparent, at least from the details available from these works.
Two notable materials under recent development are the advanced reduced-activation alloy (ARAA) and the titanium modified variety (Ti-RAFM). The former contains a small amount of Zirconium addition in a nominal 9Cr–1.2W base RAFM alloy; the latter contains Ti replacing Ta in the more common RAFM composition. As a group, these newer RAFM steels, along with Type 410 and Type 420 ferritic steels, show moderately lower permeabilities with somewhat higher activation energy than those of the more commonly used or investigated RAFM variety of steels as shown in Figure 5.
The permeability curve of Ce-ODS (a specially prepared Fe–Cr–Al alloy with cesium oxide nanoparticles) is above the other permeability curves except for the above noted Type 430 response. Also, the 1951 curve for Type 420 by Flint[16] appears to be a lower bound curve for this group of ferritic steels. As such, based on these results on the 4xx series ferritic steels, excepting the Type 430 material, and the newer variety of RAFM steels, the following tritium permeability bounds are suggested for this group of alloys:
-
Upper estimate (data): P0 = 5.60 × 10−5 mol/[m s √MPa] and Qp = 47.0 kJ/mol
-
Best estimate: P0 = 4.00 × 10−5 mol/[m s √MPa] and Qp = 48.0 kJ/mol
-
Lower estimate (data): P0 = 2.86 × 10−5 mol/[m s √MPa] and Qp = 49.0 kJ/mol
9 Comparison of Hydrogen Permeation for the Clean/Unoxidized Steel Groups
Based on the above review and evaluation of hydrogen isotopes permeation in this section (austenitic stainless steels, RAFM steels, and 400-series steels) and the preceding section (FeCrAl alloys) the resulting bounds and central data trends were estimated and described with the respective sets of permeability parameters. The estimated central trends for the tritium permeability of these steel groups are compared in Figure 6. For example, the best estimate values of tritium permeability at ~ 673 K (400 °C) are 1.5 × 10−8, 7.5 × 10−9, 7.1 × 10−9, and 1.3 × 10−9 mol/[m s √MPa], respectively, for RAFM, modified RAFM/400-series steels, FeCrAl alloys, and austenitic stainless steels.
The analysis of hydrogen permeation through the different family of steels shows that the permeability of FeCrAl alloys is generally lower than the RAFM steels and higher than the austenitic steels over the wide range of temperatures of interest, while the typical activation energy range of FeCrAl alloys is midway to those of the RAFM and austenitic steels. The estimated central trend and the bounds for the permeability of FeCrAl alloys are quite similar to those estimated for the modified or more modern RAFM steels and most of the 400-series ferritic steels, except that the bounds are wider for the FeCrAl alloys since these also include/cover the ODS variety as noted earlier.
It is interesting that the empirical estimate of the central permeability trend for the FeCrAl group is nearly identical with that of the Type 406 steel (nominal composition of 12 to 14 pct Cr and 3.5 to 4.5 pct Al by weight) which was selected earlier in this review as a reference/anchoring curve (see footnote #) for comparison between various plots subsequently developed in this work. More importantly, much earlier work of note on this particular FeCrAl alloy by Pessl[19] was briefly covered in the earlier section “What are FeCrAl Alloys?” in the Introduction.Footnote 12
10 Factors Affecting Hydrogen Permeation
10.1 Hydrogen Isotopic Effects
From the point of view of diffusion or permeation of hydrogen (protium), deuterium, and tritium through the alloys and oxides of interest the isotopic effect arises mainly from the mass difference between these three isotopes which would otherwise be expected to have similar chemical response. The latter would imply not much isotopic impact on the activation energy parameters of the diffusion or permeation process (this may not necessarily hold for adsorption/desorption process at the environmental interface). The mass effect, with vibrational frequency lower for higher mass species, leads to the diffusivity coefficient dependence to half-power of mass, but not much affecting the activation energy.[108,116,117] That is, in the case of hydrogen isotopes with deuterium to hydrogen mass ratio of close to 2, and tritium to hydrogen mass ratio of close to 3, the respective diffusivity ratios can be related by the following expression: D(H) = √2·D(D) = √3·D(T) for the alloys and the temperature range of current interest. The same expression then holds for the permeability values when the permeation is driven or controlled by the lattice diffusion of the hydrogen species,[99,103,108,118,119] implying the independence of solubility on mass vis-à-vis isotopic effect, as also confirmed in.[117]
Empirical support for the above isotope effects/relation was provided in the works reviewed, for example, for deuterium permeation in austenitic steels,[103,117,119] tritium diffusion in Type 304L steel,[118] tritium/deuterium permeation in other steels with or without oxides,[99] deuterium in martensitic steel,[108] and separation factor in permeation of the mixed hydrogen and deuterium through Type 304 and Type 316 stainless steels.[119] Although some deviation from the square-root mass dependence of diffusivity and/or permeability of the isotopes have been noted by a few investigators,[120] for the most part, the above isotopic effect has been generally observed to adequately normalize the differences in transport of hydrogen isotopes. However, the commonly used ratio for T/H of 1/√3 in the bulk metallic phases has not been confirmed for the case of diffusion in oxides where the isotopic mass related effect is expected to depend on the dominant (atomic, ionic, or molecular) form of diffusing species of T/H within the oxide.[121]
10.2 Effect of Steel and Alloys Compositional on Hydrogen Permeation
In the very early work by Flint[16] it was reported that the hydrogen diffusion rate was little affected by small variations, including additions of stabilizers, in the austenitic stainless steels. It was also noted that the diffusion rate displayed wide scatter—as much as 50 pct (or a factor of 2) range was found even under identical conditions in specimens of a single type of stainless steel. In an early review of hydrogen permeation through a wide variety of metals[122] it was noted that (a) iron and nickel display rates of hydrogen permeation between those of the highly permeable niobium and palladium and the highly resistant molybdenum and tungsten, (b) most of the iron–nickel alloys fall within a very narrow permeation range that, to a first approximation, is a weighted average of the permeation of the individual components, and (c) the iron- nickel- base alloys show approximately equivalent permeation characteristics which appear to be independent of chemical composition.
In general, it is expected that the composition of the base alloy may affect the diffusivity of hydrogen, although the sensitivity for this influence is likely to be low, i.e., a large variation in composition is likely needed to manifest any direct influence on the diffusivity. This may be inferred, e.g., considering compilations of diffusivity data[123,124] and permeability data reviewed in earlier sections[16,72,93,99,103,125,126] that show a unified diffusion response by a class of materials (austenitic, ferritic Fe–Cr, ferritic-martensitic, FeCrAl, etc.) with fairly wide variation in the class composition. This is also reflected in the permeability plots of Figures 2, 3, 4, and 5.
It is to be noted that the lowering of hydrogen permeability and measurable increase in activation energy in the case of steels containing chromium plus aluminum were related to effect of larger atomic radii of Al and Cr than Fe and their influence on the interatomic separations,[72] vis-à-vis the structural effect on passage of hydrogen even between the (bcc) ferritic steel classes. From their results of hydrogen permeability in these ferritic steel classes, determined from 1073 K down to 573 K in a cooling schedule, it was noted that the composition of ~ 12 pct Cr with 3 to 4 pct Al was optimal in lowering the permeability, with or without any redistribution of aluminum within the alloy grains observed during long annealing exposure to (reducing) hydrogen atmosphere.
The unified response of hydrogen diffusivity by a class of materials is indicative of the expected dominant role played by the atomic/crystalline structural arrangement, given a similar heat-treatment. At the same time, the relatively large uncertainty in the reported diffusion parameters which is either intrinsic to the material or inherent in the experimental techniques/analyses tends to mask any small influence of compositional variation within a class of similar alloys, adding to the overall uncertainty. However, an indirect but measurable (significant) influence of the compositional variation on the overall hydrogen permeability is apparent via its effect on the formation and characteristics of the oxides, or material’s oxidation response in general, which is the subject of a separate review on the role of oxides in tritium permeation.
11 Summary and Conclusions
A broad selection of literature related to the permeation of hydrogen isotopes was reviewed with particular reference to FeCrAl alloys and related other structural steels in nuclear reactor systems, including the fuel cladding application. The review broadly covers key aspects, factors, and data related to the tritium permeation, and includes an assessment of the data relating to the use of FeCrAl type steels as a barrier to the tritium diffusion/permeation. Key historical developments were briefly noted offering the context and perspective in support of the presented state-of-the-art in the topic of interest.
The following main conclusions and observations are made based on this review and general assessment of currently available information as cited:
-
1.
The short historical survey of the development and use of FeCrAl alloys prior to 1970 showed a great interest and incentive for these alloys in fuel cladding application, along with efforts characterizing the physicochemical and metallurgical behavior, as well as the hydrogen permeation response.
-
2.
The review of permeability data and characteristics generally support the observations that (a) the crystal structure of a clean metallic alloy has a dominant effect on the permeation response and relatively small effect of its composition in a given class of alloys, within reasonable range and similar microstructures, and (b) in the case of oxidized alloys, typically the permeation response is controlled or dominated by the oxides so that the relative performance or reduction in permeability of different alloys with similar oxide characteristics was considered to be useful for comparison at least on an order of magnitude basis or within the expected data uncertainty.
-
3.
The comparative review of hydrogen permeability in major FeCr alloys with or without aluminum yields the following general trend, from low to high resistance to permeation:
$$ \left( {\text{Unoxidized/clean}} \right) \, \{ {\text{Ferritic RAFM}} \to {\text{Fe - Cr - Al }}\left( {{\text{ODS}}} \right) \to {\text{Modified RAFM}} \cong {\text{Fe - Cr - Al}} \to {\text{Austenitic Type }}3xx\} \to \left( {{\text{Oxidized}}} \right){\text{ Fe - Cr - Al}} $$ -
4.
The ferritic steels exhibit significantly lower solubility but higher diffusivity along with lower activation energy of diffusion compared to those of the austenitic steels. This difference is attributable primarily to the bcc versus fcc crystal structure. As a group, the newer modified RAFM steels, along with Type 410 and Type 420 ferritic steels, show moderately lower permeabilities with somewhat higher activation energy than those of the more commonly used or investigated RAFM variety of steels. However, the reduction in hydrogen permeability of ferritic FeCr steels has been confirmed in several studies, associated with the addition of aluminum as an alloying element in these steels. It is seen that the permeability of FeCrAl alloys is generally lower than the common RAFM steels and higher than the austenitic steels over the wide range of temperatures of interest, while the typical activation energy range of FeCrAl alloys is in between those of the RAFM and austenitic steels.
-
5.
The activation energy for hydrogen permeation is approximately 30 pct higher in the austenitic alloys compared with the ferritic (typically ~ 50 kJ/mol in ferritic vs ~ 65 kJ/mol in the austenitic).
-
6.
The limited data on ODS variety of FeCrAl alloys in the clean/uncorroded condition generally show higher tritium permeability response compared with the non-ODS alloys. The review also shows that the permeability response is relatively less sensitive to the material composition in a given class of alloys, within reasonable range and similar microstructures.
-
7.
Based on the assessment of reviewed data and observations, reasonable central and bounding response curves were developed for tritium permeability in non-oxidized FeCrAl alloys (~12 to 22 wt pct Cr with ~ 3 to 6 wt pct Al). (Similar curves for oxidized condition are presented in a separate review paper.)
-
8.
It is interesting to note that the empirical estimate of the central trend line for the Fe–Cr–Al group (shown in Figure 6) is nearly identical with that of the Type 406 steel (nominal composition of 12 to 14 pct Cr and 3.5 to 4.5 pct Al by weight) which was selected earlier in this review as a reference/anchoring curve (see footnote #) for comparison between various plots subsequently developed in this work.
-
9.
The temperature influence is fairly well represented by the Arrhenius relation over a wide range of interest, unless (a) significant change occurs in the alloy phase or its microstructure, or (b) significant interaction occurs between the hydrogen isotope and the alloy constitution, such as hydriding or trapping. Of these only trapping seems likely to occur at lower than or near normal operating temperature in the case of FeCrAl steels generally of interest to the cladding application where, from the permeation point of view, especially under steady state condition, the simple and single Arrhenius relation is expected to be adequate, if not conservative.
Notes
An interesting observation: The words (and their pronunciation) to describe, in neutral gender, the first, the second, and the third, in the ancient language of Sanskrit are
 (prathamaṁ),
(prathamaṁ),  (dvitīyaṁ), and
(dvitīyaṁ), and  (tṛtīyaṁ), respectively. The hydrogen isotopes are commonly known as protium, deuterium, and tritium.
(tṛtīyaṁ), respectively. The hydrogen isotopes are commonly known as protium, deuterium, and tritium.The term ‘diffusibility’—originally used by Fick in 1855—may be used for better consistency, but the more commonly used term ‘diffusivity’ is maintained here.
Steady state permeation above or around the normal reactor operating temperatures is not expected to be significantly impacted by trapping; also, this review is primarily focused on the steady state response and much longer periods of the order of several years of operation. Also, in the case of ATF type FeCrAl alloys, metal hydriding is not expected to occur.
In the literature D is also labeled the diffusion coefficient.
More generally, the gradient in chemical potential or activity may be utilized in place of the concentration gradient in the above equation; this is not considered further.
A significance of this equilibrium is that it sets up the boundary condition for the diffusion equation, above Eq. [1], of no net flux at the gas–solid interface, i.e., the dissolution process at the interface is rapid enough to not hinder the diffusion downstream; as a result, the concentration gradient and flux become inversely proportional to the thickness across which the diffusivity is constant. In addition, the assumption of equilibrium at an interface is conservative from diffusion/permeation flux point of view since the actual concentration is expected to be less.
The term ‘solubility’ in these and most other cited literature is often used to mean the concentration of dissolved species in the solid at equilibrium. Notwithstanding the use of this common practice the distinction is implied, unless otherwise noted or clear by the context in this document.
For consistency and ease of comparison of data from varied sources and different units used by the authors, all data and parameters were converted to common unit of mol/[m.s.√MPa] for permeability. Where parameters were not given in the original work these were estimated graphically to best-fit the data trend or charts shown in the cited sources. Also for the same reason, and tritium evaluation being of primary interest, permeabilities displayed in all Figures are normalized or scaled by the isotopic factor (1/√3 for hydrogen and √2/√3 for deuterium data) identified by the first letter of each label (H/D/T for hydrogen/deuterium/tritium) in the included Figures. However, all parameters in the Tables retain the original values and the actual isotope used, without normalization, unless otherwise noted. Also, for expediency, the data references embedded in the labels of all Figures use the shorthand notation with the first four letters of the first author followed by the four numbers for the year of publication.
In all Figures of comparison the Arrhenius tritium permeability line for ferritic Type 406 stainless steel (with 83 wt pct Fe, 13 wt pct Cr, ~ 4 wt pct Al)[93] is included as a common anchoring line to facilitate visualizing/positioning of data between the Figures for comparisons.
The atomic packing in bcc is less compact compared to that of fcc, with the atomic packing factor of 0.68 in bcc versus 0.74 in fcc, and the passageways between the interstitials are less restrictive in bcc than in fcc structures.[97] These structural factors are expected to result in higher diffusivity in bcc compared to fcc, especially due to the lowering of activation energy for diffusion of the small elements (H, D, and T) in bcc.
Although no permeation data were included in that 1961 Pessl work[19] it is pertinent to quote from the report: “AISI 406 SS was selected as a candidate alloy because of its remarkable oxidation and corrosion resistance…of the Fe-Cr–Al base alloys evaluated, it is the only commercial grade currently available…(with) mechanical properties better than those of the regular 400 series stainless steels…(with) medium neutron capture cross section, and resistance to intergranular and stress corrosion, make these alloys especially attractive for reactor applications where their elevated temperature strength is sufficient. The outlook is very promising that AISI 406 SS, modified in its chemistry if necessary, can be used with advantage for reactor structural components and fuel cladding material in those places where austenitic stainless steels have been used or presently are being considered for use.”
References
R.B. Rebak: Accident Tolerant Materials for Light Water Reactor Fuels, Elsevier, Amsterdam, Netherlands, 2020, pp. 43–62 and 83–142.
K.A. Terrani: J. Nucl. Mater., 2018, vol. 501, pp. 13–30.
S. Bragg-Sitton, B. Merrill, M. Teague, L. Ott, K. Robb, M. Farmer, M. Billone, R. Montgomery, C. Stanek, M. Todosow, and N. Brown: Report INL/EXT-13-29957, FCRD-FUEL-2013-000264, Idaho National Laboratory, Idaho, 2014.
R.B. Rebak: Metall. Trans. E., 2015, vol. 2, pp. 197–207.
H.-G. Kim, J.-H. Yang, W.-J. Kim, and Y.-H. Koo: Nucl. Eng. Technol., 2016, vol. 48, pp. 1–15.
K. Sakamoto, M. Hirai, S. Ukai, A. Kimura, A. Yamaji, K. Kusagaya, T. Kondo, and S. Yamashita: Paper ID: A-03, Proceedings of 2017 Water Reactor Fuel Performance Meeting, Ramada Plaza Jeju, Jeju Island, Korea, September 10–14, 2017.
R.B. Rebak, K.A. Terrani, W.P. Gassmann, J.B. Williams, and K.L. Ledford: MRS Adv., 2017, vol. 2, pp. 1217–24.
R.B. Rebak: EPJ. Nuclear Sci. Technol., 2017, vol. 3. https://doi.org/10.1051/epjn/2017029.
OECD: Report No. NEA-7317, Nuclear Energy Agency (NEA), Boulogne-Billancourt, France, 2018.
K.A. Terrani, S.J. Zinkle, and L.L. Snead: J. Nucl. Mater., 2014, vol. 448, pp. 420–35.
L.J. Ott, K.R. Robb, and D. Wang: J. Nucl. Mater., 2014, vol. 448, pp. 520–33.
H.G.A. von Kantzow: U.S. Patent 717,284, Application filed December 19, 1927, Serial No. 241,259 and in Sweden December 15, 1926, Patented June 11, 1929.
S.L. Case and K.R. Van Horn: Aluminum in Iron and Steel, John Wiley and Sons Inc, New York, 1953, pp. 297–333.
E.A. Gulbransen and K.F Andrew: J. Electrochem. Soc., 1959, vol. 106, pp. 294–302.
R.B. Rebak, L. Yin, and P.L. Andresen: Corr. J., 2020. https://doi.org/10.5006/3632.
P.S Flint, The Diffusion of Hydrogen through Materials of Construction, KAPL-659, General Electric Company, Knolls Atomic Power Laboratory, Schenectady, New York, Dec. 14, 1951.
E.J. Jablonowski, F.R. Shober, and R.F. Dickerson: BMI-1230, Battelle Memorial Institute, Columbus, Ohio, Oct. 22, 1957.
J.F. Collins and J.A. McGurty: TID-17402, General Electric, Aircraft Nuclear Propulsion Department, Oct. 19, 1960.
P.J. Pessl: HW-67715, Hanford Laboratories, General Electric, Richland, Washington, February 1961.
J.W. Holladay: DMIC Memorandum 82, Defense Metals Information Center, Battelle Memorial Institute, Columbus, Ohio. Jan. 30, 1961.
R.B. Setterlund and G.R. Prescott: Corrosion., 1961, vol. 17, pp. 103–8.
W. Chub, S. Alfant, A.A. Bauer, E.J. Jablonowski, F.R. Shober, R.F. Dickerson: BMI-1298, Battelle Memorial Institute, Columbus, Ohio, Oct. 16, 1958.
S.L. Case and K.R. Van Horn: Aluminum in Iron and Steel, Wiley, New York, 1953, pp. 279–96.
S.L. Case and K.R. Van Horn: Aluminum in Iron and Steel, Wiley, New York, 1953, pp. 265–78.
T.T. Claudson and H.J. Pessl: BNWL-154, Pacific Northwest Laboratory, Richland, Washington, November 1965.
C.S. Wukusick: US Patent #3,293,007, United States Patent Office, Dec. 20, 1966.
Aerojet, IDO-28584 (Vol. I), Aerojet-General Nucleonics, San Ramon, California, Sept. 1962.
C.S. Wukusick and J.F. Collins: Materials and Research Standards, December 1964, pp. 637–46.
F.C. Robertshaw and J.L. Barton: General Electric Report GEMP-1004, United States Atomic Energy Commission, March 31, 1968.
C.G. Collins and K.M. Bohlander: US AEC, GEMP–1004, General Electric, NMPO, Nuclear Technology Department, March 31, 1968. https://doi.org/10.2172/4823602.
H.S. Edwards and K. M. Bohlander: US AEC, GEMP–1004, General Electric, NMPO, Nuclear Technology Department, March 31, 1968. https://doi.org/10.2172/4823602.
General Electric: Report GEMP-334A, Advanced Technology Services, Nuclear Materials & Propulsion Operation, United States Atomic Energy Commission, Contract No. AT (40-1) – 2847, Cincinnati, OH, Feb. 26, 1965.
F.G. Wilson, B.R. Knott, and C.D. Desforges: Metall. Trans. A., 1978, vol. 9, pp. 275–82. https://doi.org/10.1007/BF02646711.
W.J. Quadakkers, A. Elschner, W. Speier, and H. Nickel: Appl. Surf. Sci., 1991, vol. 52, pp. 271–87.
A. Strasser, J. Santucci, K. Lindquist, W. Yario; G. Stern; L. Goldstein; and L. Joseph: EPRI NP-2642, Electric Power Research Institute, Palo Alto, CA, December 1982.
L. Goldstein, O. Reyes, and A.A. Strasser: EPRI NP-5427, Electric Power Research Institute, Palo Alto, CA, April 1988.
R.B. Rebak, K.A. Terrani, and R.M. Fawcett: Paper No. PVP2016-63162, ASME 2016 Pressure Vessel & Piping Conference, PVP2016, Vancouver, BC, July 17–21, 2016. https://doi.org/10.1115/PVP2016-63162.
K. Daub, S. Persaud, R.B. Rebak, R. Van Nieuwenhove, S. Ramamurthy, and H. Nordin: in Proceedings of the 18th International Conference on Environmental Degradation of Materials in Nuclear Power Systems – Water Reactors, J.H. Jackson et al. (eds.), The Minerals, Metals & Materials Series, 2018, vol. 2, pp. 215–34. https://doi.org/10.1007/978-3-319-68454-3_19.
S.S. Raiman, K.G. Field, R.B. Rebak, Y. Yamamoto, and K.A. Terrani: J. Nucl. Mater., 2020, vol. 536, p. 152221. https://doi.org/10.1016/j.jnucmat.2020.152221.
L. Yin, T.B. Jurewicz, M. Larsen, M. Drobnjak, C.C. Graff, D.R. Lutz, and R.B. Rebak: J. Nucl. Mater., 2021, vol. 554, p. 153090. https://doi.org/10.1016/j.jnucmat.2021.153090.
B. King, J.J. Mueller, N.N. Ida, and F.G. Tate: SAE Trans., 1959, vol. 67, pp. 335–42.
D.J. Park, H.G. Kim, J.Y. Park, Y.I. Jung, J.H. Park, and Y.H. Koo: Corros. Sci., 2015, vol. 94, pp. 459–65.
B.A. Pint, K.A. Terrani, and R.B. Rebak: in Proceedings of the 18th International Conference on Environmental Degradation of Materials in Nuclear Power Systems – Water Reactors, J.H. Jackson et al. (eds.), The Minerals, Metals & Materials Series, 2018, vol. 2, pp. 235–44. https://doi.org/10.1007/978-3-319-68454-3_20.
R.B. Rebak: J. Mater., 2018, vol. 70, pp. 176–85.
C. Parisi, Z. Ma, D. Mandelli, N. Anderson, and H. Zhang: Nucl. Sci. Eng., 2020, vol. 194, pp. 748–70. https://doi.org/10.1080/00295639.2020.1732699.
C.P. Massey, K.A. Terrani, S.N. Drypondt, and B.A. Pint: J. Nucl. Mater., 2016, vol. 470, pp. 128–38. https://doi.org/10.1016/j.jnucmat.2015.12.018.
K.A. Kane, S.K. Lee, S.B. Bell, N.R. Brown, and B.A. Pint: J. Nucl. Mater., 2020, vol. 539, p. 152256. https://doi.org/10.1016/j.jnucmat.2020.152256.
K.R. Robb, M. Howell, and L.J. Ott: ORNL/SPR-2017/373, Oak Ridge National Laboratory, Oak Ridge, TN, August 2017.
R. Sweet, A. Nelson, and B.D. Wirth: ORNL/SPR-2018/995, Oak Ridge National Laboratory, Oak Ridge, TN, Sept. 12, 2018.
S.J. Zinkle, K.A. Terrani, J.C. Gehin, L.J. Ott, and L.L. Snead: J. Nucl. Mater., 2014, vol. 448, pp. 374–9.
N.M. George, K.A. Terrani, J.J. Powers, A. Worrall, and I. Maldonado: Ann. Nucl. Energy., 2015, vol. 75, pp. 703–12.
J. Locante, and D.D. Malinowski: in Tritium (CONF-71080), Eds: A.A. Moghisii and M.W. Carter, Messenger Graphics, Phoenix, AZ, 1973, pp. 35–57.
J.M Smith and R.M. Gilbert: in Tritium (CONF-71080), Eds: A.A. Moghisii and M.W. Carter, Messenger Graphics, Phoenix, AZ, 1973, pp. 57–68.
G. Jones: Fusion Sci. Technol., 2008, vol. 54, pp. 329–32.
D. Cubicciotti and R.L. Jones: EPRI NP-717, Electric Power Research Institute, Palo Alto, CA, March 1978.
E.R. Gilbert, R.P. Allen, D.L. Baldwin, R.D. Bell, J.L. Brimhall, R.G. Clemmer, S.C. Marschman, M.A. McKinnon, R.E. Page, H.G. Powers, and S.G. Chalk: Fusion Technol., 1992, vol. 21:2P2, pp. 739–44.
H.G. Yang, Q. Zhan, W.W. Zhao, X.M. Yuan, Y. Hu, and Z.B. Han: J. Nucl. Mater., 2011, vol. 417, pp. 1237–40.
D. He, Y. Lei, C. Zhang, S. Li, X. Liu, H. Zhang, Q. Lv, Y. Wu, and L. Jiang: Int. J. Hydrogen Energy., 2015, vol. 40, pp. 2899–903.
Q. Li, L.B. Mo, J. Wang, K. Yan, T. Tang, Y.C. Rao, W.Q. Yao, and J.L. Cao: Int. J. Hydrogen Energy., 2015, vol. 40, pp. 6459–64.
X. Xiang, X. Wang, G. Zhang, T. Tang, and X. Lai: Int. J. Hydrogen Energy., 2015, vol. 40, pp. 3697–707.
J. Huang, H. Xie, L.-M. Luo, X. Zan, D.-G. Liu, and Y.-C. Wu: Surf. Coat. Technol., 2020, vol. 383, p. 125282. https://doi.org/10.1016/j.surfcoat.2019.125282.
Y.M. Lyu, Y.P. Xu, H.D. Liu, X.D. Pan, H.S. Zhou, X.C. Li, Y.F. Li, Y.Y. Shan, Q. Xu, Z.S. Yang, and G.N. Luo: Int. J. Hydrogen Energy., 2019, vol. 14, pp. 18265–71.
R. Kumar: ANL-8089, Argonne National Laboratory, Argonne, IL, 1974.
A.A. Moghisii and M.W. Carter (Eds.): Tritium (CONF-71080), Messenger Graphics, Phoenix, AZ, 1973.
H. Kouts and J. Long: in Tritium (CONF-71080), Eds: A.A. Moghisii and M.W. Carter, Messenger Graphics, Phoenix, AZ, 1973, pp. 38–45.
H.D. Roehrig, R. Hecker, J. Blumensaat, and J. Schaefer: Nucl. Eng. Des., 1975, vol. 34, pp. 157–67.
T. Tanabe: J. Nucl. Mater., 2013, vol. 438, pp. S19–26.
B. Bornscheina, C. Day, D. Demange, and T. Pinna: Fusion Eng. Des., 2013, vol. 88, pp. 466–71.
J.L. Cecchi: J. Vac. Sci. Technol., 1979, vol. 16, pp. 58–70.
N. Baluc: Paper FT/1-1Rb, Conference Proceedings of The 19th International Atomic Energy Agency (IAEA) Fusion Energy Conference (FEC), held in Lyon, France, 14–19 October 2002, IAEA, Vienna, Austria, 2003.
I.L. Tazhibayeva, V.P. Shestakov, and S.T. Tukhvatulin: Paper FT/P1-4, Conference Proceedings of The 19th International Atomic Energy Agency (IAEA) Fusion Energy Conference (FEC), held in Lyon, France, 14-19 October 2002, IAEA, Vienna, Austria, 2003.
R.I. Kripyakevich, Y.I. Zvezdin, V.M. Sidorenko, I.I. Sidorak, and B.F. Kachmar: Materials Science (Springer), 1971, vol. 7, pp. 311–314. {Translated from Fiz.-Khim. Mekh. Mater., 1971, vol. 7, pp. 60–4}.
I.I. Sidorak, V.M. Sidorenko, S.K. Yaremkevich, et al.: Materials Science (Springer), vol. 10, 1974, pp. 134–6. {Translated from Fiz.-Khim. Mekh. Mater., 1974, vol. 10, pp. 22–5}.
R.E. Stickney: The Chemistry of Fusion Technology, Ed.: D.M. Gruen, Plenum Press, New York, 1972, pp. 241–319.
R.A. Strehlow and H.C. Savage: Nucl. Technol., 1974, vol. 22, pp. 127–37.
E. Serra, G. Benamati, and O.V. Ogorodnikova: J. Nucl. Mater., 1998, vol. 255, pp. 105–15. https://doi.org/10.1016/S0022-3115(98)00038-5.
R.A. Causey, R.A. Karnesky, and C. San Marchi: Comprehensive Nuclear Materials, Section 4.16, Elsevier, 2012, pp. 512–49.
X. Hu, K.A. Terrani, B.D. Wirth, and L.L. Snead: J. Nucl. Mater., 2015, vol. 461, pp. 282–91. https://doi.org/10.1016/j.jnucmat.2015.02.040.
R.B. Rebak and Y.J. Kim: Paper No. PVP2016-63164, ASME 2016 Pressure Vessel & Piping Conference, PVP2016, Vancouver, BC, July 17–21, 2016.
L.L. Lucas and M.P. Unterweger: J. Res. Natl. Inst. Stand. Technol., 2000, vol. 105, pp. 541–9.
W.L. Bryan and B.F. Dodge: AIChE J., 1963, vol. 9, pp. 223–8.
V.A. Maroni: CONF-781109-12, Department of Energy Environmental Control Symposium, Washington, D.C., Argonne National Laboratory, Argonne, IL, 1978.
A. Sieverts: Z. Phys. Chem., 1907, vol. 60U, pp. 129–201. ((In German)).
R.M. Barrer: Diffusion In and Through Solids, Cambridge University Press, London, 1951, pp. 145–206.
A. Fick: The London, Edinburgh, and Dublin Phil. Mag. J. Sci., 1855, vol. 10, pp. 30–9. (Abstracted in English). https://doi.org/10.1080/14786445508641925.
A. Fick: Ann. Phys. (Berlin, Ger.), 1855, vol. 170, pp. 59–86. (In German). https://doi.org/10.1002/andp.18551700105.
R.H. Fowler and C.J. Smithells: Proc. R. Soc. Lond. A, May 1, 1937, vol. 160, pp. 37–47.
O.W. Richardson, J. Nicol, and T. Parnell: Lond. Edinb. Dublin Phil. Mag. J. Sci., 1904, vol. 8, pp. 1–29. https://doi.org/10.1080/14786440409463168.
H.L. Eschbach, F. Gross, and S. Schulien: Vacuum., 1963, vol. 13, pp. 543–7.
C.L. Huffin and J.M. Williams: Corrosion., 1960, vol. 16, pp. 102–4.
E.H. Van Deventer and V.A. Maroni: J. Nucl. Mater., 1980, vol. 88, pp. 168–73.
E.H. Van Deventer and V.A. Maroni: J. Nucl. Mater., 1983, vol. 113, pp. 65–70.
J.T. Bell, J.D. Redman, and H.F. Bittner: J. Mater. Energy Syst., 1979, vol. 1, pp. 55–9.
W.A. Swansiger, B.E. Mills, and A.S. Nagelberg: J. Nucl. Mater., 1984, vol. 123, pp. 1292–7.
K. Sakamoto, Y. Miura, S. Ukai, A. Kimura, A. Yamaji, K. Kusagaya, T. Kondo, and S. Yamashita: Paper No. A0011 in Proceedings of TOPFUEL 2018, Prague, Czech Republic, 30 September – 04 October 2018.
Y. Urabe, K. Hashizume, T. Otsuka, and K. Sakamoto: Fusion Sci. Technol., 2020, vol. 76, pp. 392–7. https://doi.org/10.1080/15361055.2020.1712992.
L.H. Van Vlack, Elements of Materials Science – An Introductory Text for Engineering Students, Second Edition, Addison-Wesley Publishing Company, Inc., U.S.A., 1964, pp. 57–62 and pp. 247–8.
G.R. Caskey, Jr.: Hydrogen Degradation of Ferrous Alloys. Eds.: R.A. Oriani, J. P. Hirth and M. Smialowski, Park Ridge NJ: Noyes Publications, 1985, pp. 822–62.
W.A. Swansiger and R. Basatz: J. Nucl. Mater., 1979, vol. 85-86, Part P-1, pp. 335–9.
M. Matsuyama and J.D. Redman: Metall. Mater. Trans. A., 1983, vol. 14A, pp. 498–500.
J.L. Maienschein, R.G. Musket, F.E. McMurphy, and D.W. Brown: Appl. Phys. Lett., 1987, vol. 50, pp. 940–42.
S. Xiukui, X. Jian, and L. Yiyi: Acta Metall., 1989, vol. 37, pp. 2171–6.
M.R. Louthan Jr. and R.G. Derrick: Corros. Sci., 1975, vol. 15, pp. 565–77.
M.H. Armbruster: J. Am. Chem. Soc., 1943, vol. 65, pp. 1043–54.
R.I. Kripyakevich, Y.I. Zvezdin, V.M. Sidorenko, I.I. Sidorak, and B.F. Kachmar: Materials Science (Springer), 1971, vol. 7, pp. 686–688. {Translated from Fiz.-Khim. Mekh. Mater, 1971, vol. 7, pp. 47–51}.
G.W. Hollenberg, E.P. Simonen, G. Kalinin, and A. Terlain: Fusion Eng. Des., 1995, vol. 28, pp. 190–208.
D. Levchuk, H. Bolt, M. Döbeli, S. Eggenberger, B. Widrig, and J. Ramm: Surf. Coat. Technol., 2008, vol. 202, pp. 5043–7.
K.S. Forcey, D.K. Ross, J.C.B. Simpson, and D.S. Evans: J. Nucl. Mater., 1988, vol. 160, pp. 117–24.
A. Perujo, E. Serra, S. Alberici, S. Tominetti, and J. Camposilvan: J. Alloys Compd., 1997, vol. 253–254, pp. 152–5.
E. Serra and G. Benamati: Mater. Sci. Technol., 1998, vol. 14, pp. 573–8.
G.A. Esteban, A. Peña, I. Urra, F. Legarda, and B. Riccardi: J. Nucl. Mater., 2007, vol. 367–370, part-PA, pp. 473–7.
G.A. Esteban, A. Peña, F. Legarda, and R. Lindau: Fusion Eng. Des., 2007, vol. 82, pp. 2634–40.
I.V. Danilov, V.K. Kapyshev, V.G. Kovalenko, A.N. Kalashnikov, A.A. Dzhanelidze, S.A. Zhivotov, and S.B. Zlokazov: Probl. At. Sci. Technol. Ser. Thermonuclear Fusion., 2014, vol. 37, pp. 38–44.
D.W. Rudd and J.B. Vetrano, Report NAA-SR-6109, Permeability of Metals and Enameled Metals to Hydrogen, Atomics International, Oct. 30, 1961.
T. Chikada, A. Suzuki, Z. Yao, D. Levchuk, H. Maier, T. Terai, and T. Muroga: Fusion Eng. Des., 2009, vol. 84, pp. 590–2.
M.R. Louthan Jr., J.A. Donovan, and G.R. Caskey Jr.: Scr. Metall., 1974, vol. 8, pp. 643–50.
N.R. Quick and H.H. Johnson: Metall. Trans. A., 1979, vol. 10, pp. 67–70.
M.R. Louthan Jr., J.A. Donovan, and G.R. Caskey Jr.: Nucl. Technol., 1975, vol. 26, pp. 192–200.
T. Shiraishi, M. Nishikawa, T. Yamaguchi, and K. Kenmotsu: J. Nucl. Mater., 1999, vol. 273, pp. 60–5.
C. Shan, A. Wu, and Q. Chen: J. Nucl. Mater., 1991, vol. 179–181, Part-P1, pp. 322–4.
J.V. Cathcart, R.A. Perkins, J.B. Bates, and L.C. Manley: J. Appl. Phys., 1979, vol. 50, pp. 4110–9.
R.W. Webb, Report NAA-SR-10462, Permeation of Hydrogen through Metals, Atomics International, July 25, 1965.
H. Boellinhaus, H. Hoffmeister, and A. Dangeleit: Weld. World., 1995, vol. 35, pp. 83–96.
H. Boellinhaus, H. Hoffmeister, and C. Middel: Weld. World., 1996, vol. 37, pp. 16–23.
T.-P. Perng and C.J. Altstetter: Acta Metall., 1986, vol. 34, pp. 1771–81.
S. Xiukui, X. Jian, and L. Yiyi: Mater. Sci. Eng. A., 1989, vol. 14, pp. 179–87.
K.S. Forcey, D.K. Ross, and L.G. Earwaker: Z. Phys. Chem. Neue Folge., 1985, vol. 143, pp. 213–28.
R.F. Miller, J.B. Hudson, and G.S. Ansell: Metall. Mater. Trans. A., 1975, vol. 6A, pp. 117–21.
H.G. Nelson and J.E. Stein: NASA-TN-D-7265, National Aeronautics and Space Administration, Washington, DC, April 1973.
O.D. Gonzalez: Trans. Metall. Soc. AIME., 1969, vol. 245, pp. 607–12.
G.M. Grant, D.L. Cummings, and D.A. Blackburn: J. Nucl. Mater., 1987, vol. 149, pp. 180–91.
E.H. Van Deventer, V.A. MacLaren, and V.A. Maroni: J. Nucl. Mater., 1980, vol. 92, pp. 103–11.
Acknowledgments
This material is based upon work supported by the Department of Energy [National Nuclear Security Administration] under Award Numbers DE-NE0008823 & DE-NE0009047. The financial support of Global Nuclear Fuel and GE Research is gratefully acknowledged. This report was prepared as an account of work sponsored by an agency of the United States Government. Neither the United States Government nor any agency thereof, nor any of their employees makes any warranty, express or implied, or assumes any legal liability or responsibility for the accuracy, completeness, or usefulness of any information, apparatus, product, or process disclosed, or represents that its use would not infringe privately owned rights. Reference herein to any specific commercial product, process or service by trade name, trademark, manufacturer, or otherwise does not necessarily constitute or imply its endorsement, recommendation, or favoring by the United States Government or any agency thereof. The views and opinions of authors expressed herein do not necessarily state or reflect those of the United States Government or any agency thereof.
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Manuscript submitted August 26, 2021; accepted November 2, 2021.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Garud, Y.S., Hoffman, A.K. & Rebak, R.B. Hydrogen Isotopes Permeation in Clean or Unoxidized FeCrAl Alloys: A Review. Metall Mater Trans A 53, 773–793 (2022). https://doi.org/10.1007/s11661-021-06535-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11661-021-06535-8



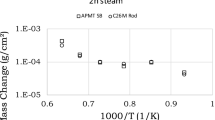







 (prathamaṁ),
(prathamaṁ),  (dvitīyaṁ), and
(dvitīyaṁ), and  (tṛtīyaṁ), respectively. The hydrogen isotopes are commonly known as protium, deuterium, and tritium.
(tṛtīyaṁ), respectively. The hydrogen isotopes are commonly known as protium, deuterium, and tritium.