Abstract
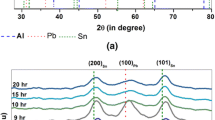
The present investigation reports the size-dependent melting behavior of bi-phasic Pb-17.5 at. pct Sb-free alloy nanoparticles, consisting of face-centered cubic (Pb) and rhombohedral (Sb) phases. These free nanoparticles (devoid of matrix) were synthesized via the solvothermal route, and subsequently different sizes were obtained by controlled heat treatment at 373 K under protective Ar atmosphere for the various durations. DSC and in situ TEM studies were used to probe the melting behavior. The experimental results reveal classical behavior, indicating the normalized eutectic temperature was inversely proportional to the nanoparticle radius. The in situ TEM investigation shows that melting initiates at the triple point among (Pb)/(Sb) and an outer surface and subsequently the melt front propagates into (Pb) phase first and then into (Sb) phase. Detailed thermodynamic modeling of the Pb-Sb system with size was carried out to decipher the size-dependent melting behavior of the biphasic nanoparticles. The experimental finding was corroborated with a theoretically predicted phase diagram as a function of size showing a reasonable match.









Similar content being viewed by others
Data Availability
The raw/processed data required to reproduce these findings cannot be shared at this time because of technical limitations during submission. The raw and processed data required to reproduce these findings will be made available to download from http://home.iitk.ac.in/~kbiswas/.
References
[1] F. Calvo: Phys.Chem.Chem.Phys., 2015, vol. 17, pp. 27922-27939.
O.V. Salata: J Nanobiotechnol., 2004, vol. 2, pp. 1-6.
[3] A. Akbarzadeh, M. Samiei, S. Davaran: Nanoscale Res. Lett., 2012, vol. 7, pp. 144.
[4] A. Patel, P. Prajapati, R. Boghra: Asian J Pharm Clin. Res., 2011, vol. 1, pp. 40-55.
S.R. Sahu, M.M. Devi, P. Mujkherjee, P. Sen, and K. Biswas: J. Nanomater., 2013, vol. 6, pp. 1–9.
K. Biswas, G. Phanikumar, K. Chattopadhyay, T. Volkmann, O. Funke, D. Holland-Moritz, and D.M. Herlach: Mater. Sci. Eng. A, 2004, vol. 375, pp. 464–67.
K. Biswas, G. Phanikumar, D. Holland-Moritz, and D.M. Herlach Chattopadhyay: Philos. Mag., 2007, vol. 87, pp. 3817–37.
P. Sharma, K. Biswas, A.K. Mondal, and K. Chattopadhyay: Scripta. Mater., 2009, vol. 166, pp. 600–03.
[5] M.M. Devi, K. Biswas: Mat. Res., 2015, vol. 18, pp. 55-60.
[6] W. Jesser, R. Shneck, W. Gile: Phys. Rev. B., 2004, vol. 69, pp. 144121.
[7] J. Sopousek, J. Vrestal, A. Zemanova, J. Bursi: J. Min. Metall. B., 2012, vol. 48, pp. 419-425.
[8] V. Bhattacharya, K. Chattopadhyay: Mater. Sci.. Eng. A, 2007, vol. 449, pp. 1003-1008.
[9] V. Bhattacharya, K. Chattopadhyay: J. Nanosci. Nanotechnol., 2007, vol. 7, pp. 1736-1743.
P.Y. Khan, V. Bhattacharya, K. Biswas, K. Chattopadhyay: J. Nanoparticle Res., 2013, vol. 15, pp. 1-15
[11] P.Y. Khan, K. Biswas: Philos. Mag., 2014, vol. 94, pp. 2031-2045.
[12] P.Y. Khan, K. Biswas:J. Nanosci. Nanotechnol., 2015, vol.15, pp. 309-316.
K. Chattopadhyay, V. Bhattacharya: Encyclopedia Nanosci. Nanotechnol., 2004, pp. 217–32.
[14] G. Allen, W. Jesser: J. Cryst. Growth, 1984, vol. 70, pp. 546-551.
[15] S. Ali, V. Myasnichenko, E. Neyts: Phys. Chem. Chem. Phys., 2016, vol.18,pp.792-800.
[16] T.E. Song, G. Wilde, M. Peterlechner: Applied Physics Letters, 2014, vol.105, pp. 241902-1-5.
N. Oehl, P. Michalowski, M. Knipper, J. Kolny-Olesiak, T. Plaggenborg, J.R. Parisi: J. Phys. Chem. C, 2014, vol.118, pp. 30238-43.
[18] S. Bajaj, M.G. Haverty, R. Arróyave, S. Shankar: Nanoscale, 2015, vol. 7, pp. 9868-9877.
[19] B. Kim, J. Tersoff, C.-Y. Wen, M. Reuter, E. Stach, F. Ross: Phys. Rev. Lett., 2009, vol. 103, pp. 155701-1-155701-4
[20] J. Park, J. Lee: Calphad, 2008, vol. 32, pp. 135-141.
[21] A. Shirinyan, M. Wautelet: Mater. Sci. Eng. C, 2006, vol. 26, pp. 735-738.
[22] Y. Dahan, G. Makov, R. Shneck: Calphad, 2016, vol. 53, pp. 136-145.
[23] N. Braidy, G.R. Purdy, G.A. Botton: Acta Mater., 2008, vol.56, pp. 5972-5983.
[24] T. Massalski: Binary Alloy Phase Diagrams, 3rd ed., ASM International, Metals Park, OH, 1992, pp. 206-210.
M.M. Devi and K. Biswas: Mater. Chem. Phys., 2015, vol. 166, pp. 207-14
G. Wilde, P. Brunzel, H. Rössner and J. Weissmüller, Phase Equilibria of Nanoscale Metals and Alloys. In: D.M. Herlach (eds) Phase Transformations in Multicomponent Melts. Wiley, Weinheim, 2008, pp. 87-106
[27] D.A. Porter, K.E. Easterling, M. Sherif: Phase Transformations in Metals and Alloys, 3rd edition, CRC press, NY, USA, 2009, pp. 31-41
[28] V. Bhattacharya, E. Yamasue, K. Ishihara, K. Chattopadhyay: Acta Mater., 2005, vol. 53, 4593-4603.
[29] J. Lee, H. Mori: Vac. Sci. Technol. A, 2003, vol. 21, pp. 32-36.
V. Bhattacharya, K. Chattopadhyay: Mater. Sci. Eng. A, 2004, vol. 375, pp. 932-935.
J.G. Lee, H. Mori: Eur. Phys. D Atomic Mol. Opt. Plasma Phys., 2005, vol. 34, pp. 227-30.
[32] P. Bhattacharya, V. Bhattacharya, K. Chattopadhyay: J. Mater. Res., 2002, vol. 17, pp. 2875-2883.
[33] J. Weissmüller, P. Bunzel, G. Wilde: Scr. Mater., 2004, vol. 51, pp. 813-818.
[34] A.T. Dinsdale, Calphad, 1991, vol. 15, pp. 317-425.
[35] S. Hassam, D. Boa, Y. Fouque, K. Kotchi, J. Rogez: J. Alloy. Comp., 2009, vol. 476, pp. 74-78.
[36] H.M. Cundy, A.P. Rollett: Mathematical models, Clarendon Press, Oxford, U.K., 1961, pp. 161
[37] W. Qi, M. Wang: Mater. Chem. Phys., 2004, vol. 84, pp. 280-284.
[38] L. Mondolfo, N. Parisi, G. Kardys:Mater. Sci. Eng., 1985, vol. 68, pp. 249-266.
[39] J. Butler: Proceedings of the Royal Society of London. Series A, 1932, vol. 135, pp. 348-375.
[40] R. Picha, J. Vřešťál, A. Kroupa: Calphad, 2004, vol. 28, pp. 141-146.
[41] M. Ghasemi, Z. Zanolli, M. Stankovski, J. Johansson: Nanoscale, 2015, vol. 7, pp. 17387-17396.
[42] C. Chen, J.-G. Lee, K. Arakawa, H. Mori: Appl. Phys. Lett., 2011, vol. 99, pp. 013108-1-013108-3.
[43] C. Chen, J.-G. Lee, K. Arakawa, H. Mori:Appl. Phys. Lett., 2011, vol. 98, pp. 083108.
[44] O. Redlich, A. Kister: Ind. Eng. Chem. Res., 1948, vol. 40, pp. 345-348.
T. Tanaka: Mater. Sci. Forum, 2010, vol. 653, pp. 55-75.
[46] V. Kumikov, K.B. Khokonov: J. Appl. Phys., 1983, vol. 54, pp. 1346-1350.
[47] R. Speiser, D. Poirier, K. Yeum: Scr. Mater., 1987, vol. 21, pp. 687-692.
[48] K. Yeum, R. Speiser, D. Poirier: Mater. Sci. Eng. B, 1989, vol. 20, pp. 693-703.
Acknowledgments
Authors would like to thank Prof. K. Chattopadhyay and Dr. S. Kashyap for helping in the in situ TEM measurement. Authors would like to thank Advance Imaging Centre at IIT Kanpur and office of Dean R&D for microscopic facilities.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Manuscript submitted October 8, 2018.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Appendix
Appendix
1.1 Details of Thermodynamics Calculations
The objective of the appendix is to describe the details and procedure for obtaining the nanophase diagram using a thermodynamic database of molar volume and surface tensions of the respective pure metals. The method is adapted from the model developed by Weissmuller et al.[37]
The molar free energy, Gm, of a regular solution is commonly derived as:
where \(x \; {\text{indicates }}\)the mole fractions of components and \(^\circ G_{\text{Pb}}\) and \(^\circ G_{\text{sb}}\) are the standard Gibbs free energies of the pure components Pb and Sb, respectively. T is the temperature, R is the universal gas constant, and \(G_{mix}^{xs}\) is the excess Gibbs free energy of mixing, which is extended by the Redlich-Kister formalism[48] as:
where \(\varOmega_{\vartheta }\) is a non-ideal interaction parameter. \(\vartheta\) is the order of expansion where for regular solution phases \(\vartheta\) = 0 and \(\vartheta\) = 1 for non-regular solution phases. Thermodynamic functions used during calculation are tabulated in Table IV.
To evaluate the surface energies of solid phases (\(\sigma^{\alpha}\) and \(\sigma^{\beta }\)) by using Butler’s equation, the molar surface area, surface energy and partial excess free energy of the pure component Pb or Sb need to be determined first. Therefore, the molar surface area can be calculated by using the following equation, \(A_{i} = 1.091 N_{\text{A}}^{{\frac{1}{3}}} \left( {V_{i} } \right)^{{\frac{2}{3}}}\), where \(N_{\text{A}}\) and \(V_{i}\) are Avogadro’s number and the molar volume of component i. The molar volume\(V\) is estimated by:
\(V_{\text{Pb}}\) and \(V_{\text{sb}}\)are the molar volumes of the pure components of Pb and Sb. The values of the molar volume of pure Pb and Sb in liquid state from the reported literature were used.[45,49] For evaluating the molar volume of pure components Pb and Sb in solid state, the following equation was used:
where \(\alpha_{x}\) is the thermal expansion coefficient, which is a ratio of the change in volume of solid while melting, \(\alpha_{x} = \frac{{V_{\text{x, m.p}}^{\text{L}} - V_{\text{x, m.p}}^{\text{s}} }}{{V_{\text{x, m.p}}^{\text{s}} }}\), where, \(V_{\text{x, m.p}}^{\text{s}}\) and \(V_{\text{x, m.p}}^{\text{L}}\) are the molar volumes of pure liquid and solid x (Pb or Sb) at melting temperature.
Furthermore, the surface energy values of pure Pb and Sb in liquid state were obtained directly from the literature.[44] Some of the reported references showed the surface energy value of the pure solid at the melting point is observed to be 25 pct larger than surface energy of the pure liquid.[49,50] Therefore, Eq. (A5) was used to approximate the surface energy of the pure solid component:
where \(\sigma_{x,m.p}^{\text{L}}\) is the surface energy of pure liquid x (Pb or Sb) at its equilibrium melting point Tx,m.p..
All the reported and calculated temperature-dependent surface energies and molar volumes of the pure components (Pb, Sb) in the solid as well as liquid state are summarized in Table III.
The partial excess Gibbs free energy of components Pb and Sb in the bulk can be evaluated by Reference 49:
For obtaining the partial excess Gibbs free energy of components Pb and Sb in the surface, Speiser, Yeum’s model has been used. According to Yeum’s model.[51,52]
\(\varOmega^{mix} = 0.85\); liquid alloy and \(\varOmega^{mix} = 0.84\); solid alloy
\(\varOmega^{mix}\) represents the ratio of the coordination number in the surface to that in the bulk.[49]
The surface energies of solid phases (\(\sigma^{\alpha }\) and \(\sigma^{\beta }\)) can be calculated by setting all the values in Butler’s Eqs. [A6a] and [A6b] computed by solving Eqs. [A3] through [A7a]. The equations are simultaneous equations with two unknowns: \(x_{Pb,Sb}^{Surface}\) and\(\gamma_{\alpha ,\beta }.\) Furthermore, these equations can be solved for those unknowns numerically.
The four possible states and corresponding free energy functions are given by\(G^{{\text{total}},\alpha \beta },\)\(G^{{\text{total}},\alpha l},\)\(G^{{\text{total}},\beta l}\) and \(G^{{\text{total}},l}.\) These four free energies were calculated as a function of the phase fraction at each temperature. Let us assume p1 and p2, the terminal mole fraction of the Pb- and Sb-rich phases, respectively. Here, p1 and p2 correspond to the intercepts of a common tangent with the free energy curve. To approximate the two minima of the Gibbs free energy curves, the following coupled equations are utilized:
p1 and p2 are solved for ranges of temperature and plotted on a phase diagram as shown in Figure 8.
Rights and permissions
About this article
Cite this article
Devi, M.M., Tiwari, K. & Biswas, K. Size-Dependent Melting Behavior of Pb-17.5 At. Pct Sb-Free Biphasic Alloy Nanoparticles. Metall Mater Trans A 50, 3959–3972 (2019). https://doi.org/10.1007/s11661-019-05275-0
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11661-019-05275-0