Abstract
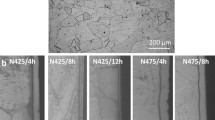
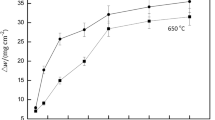
Low-temperature nitridation is a widely used surface heat treatment. Low-temperature liquid nitridation was applied to 316 austenitic stainless steel and an S-phase (expanded austenite) layer was achieved on the alloy surface. The effect of the S-phase layer on corrosion resistance and stress corrosion cracking was investigated in a sour environment. When a bending stress of 164 MPa (80 pct yield stress, YS) was applied, no macroscopic corrosion cracking and pits were observed on the nitrided samples and the S-phase layer stayed intact. Although no macroscopic corrosion cracking was observed on the non-nitrided samples under 205 MPa (100 pct YS), some pits were formed on the alloy surface. This could be attributed to the high stresses and hardness, and the excellent corrosion resistance of the S-phase layer introduced by low-temperature nitridation. Supersaturated nitrogen atoms in the S-phase layer can effectively prevent the decrease in pH of the corrosive medium and accelerate the alloy repassivation kinetics. However, when the bending stress was increased to 205 and 246 MPa (100 pct YS, 120 pct YS), macroscopic cracks were observed in the presence of both tensile stress and a corrosive medium.












Similar content being viewed by others
References
A. Tomio,M. Sagara,T. Doi,H. Amaya, N. OtsuKa. Corros. Sci.,2015, 98: 319-398.
[2]W. Liang, X.L. Pang, and K.W. Gao: Corros. Sci., 2016, vol.111,pp.637-648.
[3]W.J. Yang, M. Zhang, Y.H. Zhao, M.L. Shen,H. Lei,L. Xu,J.O. Xiao,J. Gong, B.H. Yu, and C. Sun: Surf. Coat. Tech., 2016, vol.298,pp.64-72.
[4]M. Laleh, F. Kargar, and M. Velashjerdi: J. Mater. Eng. Perform., 2013, vol.22,pp.1304-1310.
[5]H. Wang,P. Zhou,S. Huang, and C. Yu: Int. J. Electrochemi. Sci., 2016, vol.11,pp.1293-1309.
[6]J. Fliethmann, H. Schlerkmann, and W. Schwenk: Corros. Sci., 1992, vol.24,pp.746.
M. Ueda: EP Patent, no. 0851037, 2002.
[8]G.O. Lauvstad, R. Johnsen, O. Borck, E.F.D. Silva,and J. Walmslse: Corrosion,11-15 March,NACE International,Nashville Tennessee,2007,pp.076601-15.
[9]L.W. Tsay, M.Y. Chi, H.R. Chen, and C. Chen: Mater. Sci. Eng. A, 2006, vol.416, pp.155-160.
[10]K.G. Solheim, J.K. Solberg, J. Walmsley,F. Rosenqvist,and T.H. Bjørnå: Eng. Fail. Anal., 2013, vol.34, pp.140-149.
[11]W.T. Tsai, and S.L. Chou: Corros. Sci., 2000, vol.42,pp.1741-1762.
[12]E.B. Hansson,M.S. Odziemkowski,R.W. Gillham: Corros. Sci., 2006, vol.48,pp.3767–3783.
[13]M. Castillo, H. Rincon, S. Duplat, E. Baron, and J. Vera: Corrosion, NACE International,Orlando, Florida, USA,2000
[14]W. He, O. Knudsen, and S. Diplas: Corros. Sci., 2009, vol.51,pp.2811-2819.
[15]K. Funatani: Met. Sci. Heat. Treat., 2004, vol.46, pp.277-281.
J.R. Easterday and J.F. Pilznienski: US Patent, no. 6746546, 2004.
[17]Sun Y, and Bell T: Wear, 1998, vol.218,pp.34-42.
Dong H. Int. Mater. Rev., 2010, 55(2):65-98.
[19]X.Y. Li, H. Dong: Mater. Sci. Tech. Ser, 2003, vol.19,pp.1427-1434.
K.A. Moyer: US Patent, no. 8182617, 2012.
D.Z. Zeng, N.Y Zhang, G. Tian, J.Y. Hu, Z. Zhang, T.H. Shi. Jou. Wuhan Univ. Technol., 2015, 30(2):397-403.
[22]Y. Sun, X.Y. Li, T. Bell: J. Mater. Sci., 1999, vol.34,pp.4793-4802.
[23]W. Shi, X.Y. Li, and H. Dong: Wear, 2001, vol.250,pp.544-552.
D Manova, G Thorwarth, S Mändl, H Neumann, B Stritzker, B. Rauschenbach. Nuclear Instrum. Methods (2006) 242: 285-288.
[25]H. Dong, M. Esfandiari, and X.Y. Li: Surf. Coat. Technol., 2008,vol. 202,pp.2969-2975
MK Lei, ZL Zhang. J. Vac. Sci. Technol. A (1997) 15: 421-427.
[27]D. Li, W. Hao, Y. Liu, G. Lu: Corrosion, NACE International,San Antonio,Texas,USA, 2014
D Wang, F Ernst, H Kahn, AH Heuer. Metall. Mat. Trans. A (2014) 45(8): 3578-3585.
SP Brühl, R Charadia, S Simison, DG Lamsa, A Cabo. Surf. Coat. Tech. (2010) 204: 3280-3286.
[31]M.T. Shehata, M. Elboujdaini, and R.W. Revie: Safety, Reliability and Risks Associated with Water, Oil and Gas Pipelines. Springer Netherlands, 2008,pp.115-128
[32]Y. Jiang, J.M. Gong, Z.G. Yu, and D.S. Rong: Key Eng. Mat., 2011, vol.479,pp.34-39.
E. Anelli, M. Armengol, P. Novelli, and F. Tintori: US Patent, no. 9598746, 2017.
R.B. Frandsen, T. Christiansen, M.A.J. Somers. Surf. Coat. Tech., 2006, 200: 5160-5169.
M. Monnot, R.P. Nogueira, V. Roche, G. Berthomé,E. Chauveau,R. Estevez, M. Mantel. Appl. Surf. Sci., 2017, 394: 132-141.
[36]G.V. Boven, W. Chen,R. Rogge: Acta Mater., 2007, vol.55,pp.29-42.
[37]W.Chen, G.V. Boven, R. Rogge: Acta Materialia, 2007, vol.55,pp.43-53.
[38]A.H. Heuer, F. Ernst, H. Kahn,A. Avishai,G.M. Michal,D.J. Pitchure,and R.E. Ricker: Scripta Mater., 2007, vol.56,pp.1067-1070.
[39]D. Hoeft, B.A. Latella, and K.T. Short, and J. Phys.: Condens. Matter.,2005, vol. 17, pp. 3547–58.
[40]C.X. Li and T. Bell: Corros. Sci., 2004, vol. 46, pp. 1527–47.
J. Wang, Y.H. Lin,M. X. Li,H.Y. Fan,D.Z. Zeng, J. Xiong: Metall. Mat. Trans. B, 2013, 44(4): 1010-1016.
[42]D. Wang, H. Kahn,J.J Lewandowski,F. Ernst,G.M. Michal,and A.H. Heuer: Mat. Sci. Eng. A, 2012, vol.556,pp.43-50.
[43]Z.Y. Liu, C.F. Dong, X.G. Li, Q. Zhi, and Y.F. Cheng: J. Mater. Sci., 2009, vol.44,pp.4228-4234.
Y.T. Xi, D.X. Liu, D.Han. Surf. Coat. Tech., 2008, 202: 2577-2583.
[45]T. Sunaba, T. Shinohara, Y. Miyata, S. Asakura,T. Yakou,and Y. Tomoe: Zairyo-to-Kankyo, 2014, vol.63,pp.468-474.
K. Osozawa, and N. Okato: Passivity and its Breakdown on Iron and Iron Based Alloys, USA-Japan Seminar, Honolulu, NACE, Houston, TX, 1976, pp.135.
[47]C. O. A. Olsson: Corros Sci, 1995, vol.37(3),pp.467-479.
Y. Fu, X.Q. Wu, E. H. Han, W.K. Ke,K. Yang, Z.H. Jiang: Electrochim. Acta, 2009. 54(5): 1618-1629.
P.R. Levey, and A.R.V. Bennekom: Corrosion, 1995, 51: 911-921.
[50]Y.C. Lu, M.B. Ives, and C.R. Clayton: Corros. Sci., 1993, vol.35,pp.89-96.
R.C. Newman, T. Shahrabi. Corros. Sci., 1987, 27(8): 827-838.
[52]M. Tsujikawa, D. Yoshida, N. Yamauchi, N. Ueda,T. Sone,and S. Tanaka: Surf. Coat.Tech., 2005, vol.200,pp.507-511.
Acknowledgments
The authors are very grateful to the grant of National Natural Science Foundation of China (Nos. 51471112 and 51611130204) and Science and Technology Planning Project of Sichuan (No. 2016GZ0173) for the financial support of this research. The author (JW) would like to thank H. Dong at the University of Birmingham, UK, for his valuable discussion during the course of the research and writing.
Author information
Authors and Affiliations
Corresponding author
Additional information
Manuscript submitted May 4, 2017.
Rights and permissions
About this article
Cite this article
Zhang, X., Wang, J., Fan, H. et al. Stress Corrosion Behavior of Low-temperature Liquid-Nitrided 316 Austenitic Stainless Steel in a Sour Environment. Metall Mater Trans A 49, 356–367 (2018). https://doi.org/10.1007/s11661-017-4382-5
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11661-017-4382-5