Abstract
The microstructural features, physicochemical, thermal, and mechanical properties of eutectic SnZn alloys with varying Ga contents were examined in this study. In the microstructure study using scanning electron microscope and X-ray diffraction analysis, intermetallic phases were not observed. The results indicated that the primary effect of Ga was increased mechanical properties. The physicochemical properties of liquid alloys, density, viscosity, and surface tension were measured using the free flow method. The addition of Ga to eutectic SnZn alloy generally reduced density, surface tension, viscosity, and melting point, and increased the coefficient of thermal expansion and electrical resistivity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Reducing the melting temperature is one of the objectives in finding a replacement for lead-free solders. The melting temperature of eutectic SnPb is 456 K (183 °C), which compares to alternatives as follows: SnZn 471 K (198 °C), Sn-Cu-Ag 490 K (217 °C), SnAg 494 K (221 °C), and Sn-Cu 500 K (227 °C), as presented by Zhang and Tu.[1] As shown in the preliminary study performed by Reference 2, the melting point is reduced with the addition of Ga to SnZn alloys. In the same study, the authors showed an increase of the wetting rate and a reduction of wetting angle with increasing addition of Ga. Chen et al.[3] obtained similar data for the wetting test on Cu substrate using SnZn alloys with Ga, observing an increasing spreading area, but only to 0.5 Ga. For 1.0, 2.0, and 3.0 Ga the spreading area values were the same. In the soldering process, the thickness of intermetallic phases (IMPs) at the interface of the solder and Cu substrate with the addition of Ga to alloys increased. Song et al.[4] studied the microstructure, thermal, and tensile properties of SnZn-Ga alloys to 1.8 wt pct Ga content. Reducing melting temperature and increasing tensile strength with increasing addition of Ga to eutectic SnZn were shown.[4] Xue et al.[5] investigated the influence of Pr addition to SnZn (0.5 Ga), and the obtained IMPs layer thickness for an SnZn (0.5Ga) alloy on Cu substrate was similar to that found by previous authors.[3] The addition of Ga, which dissolves in eutectic SnZn alloys as observed in Reference 4, caused increasing tensile strength, and similar effect was obtained for the addition of Na to SnZn alloys. However, in the latter case, increasing tensile strength was indicated for precipitates of IMPs Na-Sn and Na-Zn.
The aim of this study is to identify the characteristics of SnZn eutectic alloys with Ga additions (0.1, 0.2, 0.5, 1.0, and 3.0 wt pct). The density, viscosity, and surface tension were obtained using the free flow method. Differential scanning calorimetry (DSC) measurements were taken to determine the melting point, then the specified linear expansion was determined by dilatometry and resistivity measurements, using the four-point probe method. The characteristic microstructures of the obtained cast alloys were also observed by SEM and analysis of the occurring phase using X-ray diffraction (XRD) analysis, and a mechanical test was carried out to show the positive effect of Ga addition to eutectic SnZn alloys.
2 Experimental
High purity metals Sn, Zn, and Ga (99.999 pct), were used for the preparation of the alloys. Samples were melted in a furnace placed in a glove box with high purity argon, to provide the best possible protective atmosphere during the experiment. The level of O2 and H2O was below 1 ppm as measured by solid-state analyzers for oxygen and moisture, and the N2 content was lowered (although it was not monitored), owing to Ar gas circulating through the high temperature purification system filled with Ti shavings heated to 1123 K (850 °C). In order to prepare samples for electrical resistivity measurements, the liquid alloys were sucked into quartz capillaries of 2 mm uniform inner diameter, in which they solidified rapidly.[6] The equipment and experimental procedure for DSC, thermo-mechanical analysis (TMA), electrical resistivity, and mechanical tests were described.[6] To measure the physicochemical properties of SnZnGa alloys, the discharge crucible method (DC)[7] was used. The free outflow of molten metal occurred through an orifice of specified diameter in the bottom of a crucible, and from a measurement of the mass of molten metal flowing out of the crucible as a function of time, the surface tension, density, and viscosity could be calculated simultaneously, from one experiment. Calibration involved the use of a crucible with a hole, and the parameters specifying the experiment were obtained for pure metals Sb, Sn, and Zn.[8] The liquid metal temperature was controlled with a thermocouple placed inside the crucible and connected to a temperature controller. The equipment for the DC method was constructed and placed in a glove box with high purity argon, to provide the best possible protective atmosphere during the experiment. The microstructure characteristics and elemental analysis were analyzed using scanning electron microscopy (SEM) coupled with energy dispersive spectrometry (EDS), and XRD to improve the occurring phases. Mechanical tests were conducted on an INSTRON 6025 testing machine modernised by Zwick/Roell. The conditions of testing were in accordance with EN ISO 6892-1:2009, with a strain rate of 0.00025 (1/s) at room temperature. The tensile and compressive tests were performed for each of the SnZn alloys with Ga additions, and three successful measurements were taken. The samples for tensile tests were cast to graphite molds (Ø8 × 100 mm) with the base measuring 5 mm diameter and 40 mm length.
3 Results and Discussions
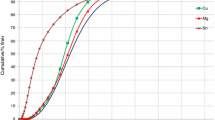
The DSC curves of SnZn alloys with Ga addition are presented in Figure 1. With increasing Ga addition to eutectic SnZn, the melting temperature reduced and the same results were obtained.[2,4] Song et al.[4] recorded a melting point of 468 K (195 °C) for 1.8 Ga, compared to 472K (199 °C) for 2 (wt pct) Ga obtained by Reference 2. But Zhang et al.,[2] for alloys with higher Ga content, recorded a greater decrease of the melting point [468 K, 461 K, 447 K, and 428 K (195 °C, 188 °C, 174 °C, and 155 °C) for 3, 4, 6, and 8 (wt pct) Ga, respectively]. According to the phase diagram of Ga-Sn-Zn,[2] which is built from the binary eutectic components, of E1 Sn8.8Zn [471.5 K (198.5 °C)], E2 Ga15Zn (292.0 K (19 °C)), and E3 Ga4Sn (298 K (25 °C)). In this ternary system, the eutectic points formed a triangle that can be described as a pseudo-binary eutectic Ga-Sn8.8Zn section, and the calculated eutectic point of the ternary system is 283 K (10 °C).[2] For the samples with 3.0 (wt pct) addition of Ga, the regions of high Ga concentration with Sn or Zn start to dissolve from 323 K (50 °C), and this reaction reaches its maximum at 392 K (119 °C). The same behavior was observed in thermal expansion coefficient (CTE) and electrical resistivity measurements.
Physical data on density, viscosity, and surface tension were obtained from the DC method.[7] The method consists of allowing the free flow of the liquid alloy through a hole in the bottom of the crucible, to a second crucible which is on a set of scales. The changes of mass vs time data obtained from the experiment, and use of the numerical method, allow Eq. [1] to be solved and give all three properties.[7,8]
where ρ density of liquid (kg/m3), g gravitational acceleration (m/s2), r 0 orifice radius in the bottom of crucible (m), σ surface tension (mN/m), Q free flow (m3/s), η viscosity (mPa s), a 1, a 2, and a 3 are constants in the polynomial describing C d vs Re, C d discharge coefficient determined for a given crucible based on the free flow of liquids with known density, Re Reynolds number.
Density, viscosity, and surface tension are presented in Figure 2. The properties of pure Ga at 623 K (350 °C) (density 5.921 (g/cm3), surface tension 696.0 (mN/m), and viscosity 0.942 (mPa s)) were taken from Reference 9. Compared to eutectic SnZn 6.900 (g/cm3), 546.1 (mN/m), 1.652 (mPa s),[10] the addition of Ga to SnZn, caused a reduction of surface tension, density, and viscosity. As expected, the density and viscosity of SnZnGa alloys are lower than those of eutectic SnZn,[10] whereas a decrease of surface tension [about 2 pct in the case of alloy containing of 3 (wt pct) of Ga] is not uncommon, because of the similar effect of a small addition of high surface tension component, as observed earlier in the case of Ga-Sn.[11] In the Ga-Sn and Ga-Zn system,[11] negative deviations from the ideal solutions were observed. In the SnZn with Ga doped to 3.0 (wt pct), surface tension slightly reduced with Ga addition, as shown in Figure 2(b). Reduction of density (Figure 2(a)) and viscosity (Figure 2(c)) for SnZn with Ga, are caused by dissolved Ga in eutectic SnZn, which shows on the microstructure of the cast alloys (Figure 3), and is also indicated in phase diagrams of Ga-Sn, Ga-Zn[14] and Ga-Sn-Zn.[2] In the case of the SnZn + Na system, viscosity increased with the addition of Na, caused by the creation of IMPs precipitates. The obtained density, surface tension, and viscosity for SnZn + Ga were lower compared to the literature data for eutectic PbSn 8.127 (g cm−3), 495 (mN m−1),[12] and 1.864 mPa s[13] respectively, at a temperature of 623 K (350 °C). Temperature dependencies of density, surface tension, and viscosity are presented in Table I. The values obtained were described by the linear and Arrhenius equations, and the parameters of the equation presented in the table, along with the density, surface tension, and viscosity values, are calculated for a temperature of 623 K (350 °C).
The microstructure of the casting alloys are presented in Figure 3 for (a) 0.1, (b) 0.2, (c) 0.5, (d) 1.0, and (e) 3.0 (wt pct) content Ga in SnZnGa alloys. For 3.0 (wt pct) Ga, the secondary electron micrographs and EDS elemental maps for Ga, Sn, and Zn were done. On the EDS maps, the dispersion of Ga in the entire volume of the sample is shown. In Table II, the EDS analysis obtained for SnZn with 1.0 (wt pct) Ga, presenting Ga occurrence in the Sn and Zn region, is shown. The XRD analyses are shown in Figure 4. EDS analysis revealed the dissolution of Ga in Sn and Zn, which is in agreement with the phase diagram of Ga-Sn and Ga-Zn.[14] Comparing the obtained microstructure with the literature, such as Song et al.[4] and Chen et al.,[15] the Ga content to 3.0 pct does not change the structure of the eutectic SnZn.
Electrical resistivity is presented in Figure 5, for (a) temperature dependences of SnZn alloys doped with Ga, and (b) the influence of the addition of Ga to SnZn alloys at 373 K (100 °C) for electrical resistivity. The influence of Ga on SnZn alloys means that a small addition of Ga reduces electrical resistivity [up to 0.5 (wt pct) Ga], and after that point increases it. The reducing of electrical resistivity is related to the solubility limit of Ga in Sn and Zn according to phase diagrams of Ga-Sn and Ga-Zn.[14] Beyond this limit, the electrical resistivity increases with increasing Ga content in alloys. In the case of SnZn with In,[16] the electrical resistivity increases with the addition of In to alloys. All received data for SnZn + Ga alloys show that electrical resistivity is higher compared to eutectic SnZn,[16] but lower compared to eutectic Sn37Pb.[16] For the addition of 3.0 (wt pct) Ga, three temperature ranges in the slope of the electrical resistivity line were observed. Changes of the same character were observed for linear expansion for SnZn + 3Ga (wt pct). The electrical resistivity of eutectic Sn37Pb (wt pct) alloy is 14.50 (μΩ cm), which is very high compared to eutectic SnZn alloy 11.70 (μΩ cm) and SnZn with Ga from 12.09, 12.03, 12.02, 12.86, and 14.41 (μΩ cm) at 298 K (25 °C). This lower value leads to reduced loss of energy and increased efficiency, thus expanding the possibility of the application of SnZn with Ga in industry.
Linear expansion for SnZn alloys with Ga is presented in Figure 6, and the CTE in Table III. The different trends obtained for SnZn + Ga is compared to SnZn + In.[16] In the literature, linear expansion data increase with the addition of In. For CTE, this expansion increases up to 1.0 (wt pct) Ga, and reduces for 3.0 (wt pct) Ga. The solubility limit of Ga in Sn and Zn has an influence on linear expansion, as shown on the phase diagram.[2] The 3 (wt pct) of Ga is very close to the chemical content in SnZnGa, when observed as a liquid at room temperature. The linear thermal expansion vs temperature for SnZn + 3.0Ga (wt pct) alloy show changes to the slope, which has an impact on the value of CTE. The same changes were observed in electrical resistivity and DSC measurement, indicating that surplus Ga leads to changes of these properties.
The effect of Ga addition to eutectic SnZn on mechanical properties can be seen from the strain–stress curves shown in Figure 7. The ultimate tensile strength and elongation values are summarised in Table IV. The Rp0.2 (plastic strain of 0.2 pct), R m (tensile strength), and A5.65 (percentage elongation after fracture) were obtained from tensile testing. The Ga additions caused increasing tensile strength, but at the same time elongation was reduced. The tensile strength of the Sn-37Pb, Sn-9Zn, Sn-9Zn-0.5Ag, Sn-9Zn-0.5Al, and Sn-9Zn-0.5Ga (wt pct) were 55, 78, 62, 73, and 78 MPa, respectively, according to data obtained by Chen et al.[15] Comparing the literature data[15] with the results obtained from the experiment, the tensile strength for 1.0 and 3.0 (wt pct) Ga are higher. All of the SnZnGa alloys showed higher values of tensile strength than Sn-37Pb.[15] The addition of Ga to SnZn reduced the elongation very quickly. For 0.5 (wt pct) Ga this was 16.8 pct, while the literature data on elongation for Sn-37Pb, Sn-9Zn, Sn-9Zn-0.5Ag, Sn-9Zn-0.5Al, and Sn-9Zn-0.5Ga were 38, 41, 37, 38, and 41 pct, respectively.[15] Although the obtained data and[15] value of tensile strength for SnZn with 0.5 Ga are almost the same, the elongation value is a factor of the two smaller. Song et al.[4] obtained elongation of more than 50 pct for SnZn alloys with Ga addition to 1.0 (wt pct), and recorded reduced elongation to ~30 pct for 1.8 Ga, but the tensile strength was similar to that presented in this work. The various results obtained for elongation could be due to different preparations of alloys, even for similar morphologies, which were obtained by References 4 and 15. Osorio et al.[17] show the influence of preparation of samples for obtained stress and elongation for eutectic SnAg, SnBi, SnZn, SnPb alloys. The most sensitive was eutectic SnZn, for which the obtained stress value ranged from ~41 to ~59 (MPa), and strain from 50 to 26 pct. This study and Reference 4 confirm the occurring trend of increasing tensile strength with the addition of Ga to SnZn. From the viewpoint of SnZnGa alloys as a replacement for lead solders, increasing the stress will raise the strength of soldered connections.
4 Summary
The addition of Ga to a eutectic SnZn alloy generally reduced density, surface tension, viscosity, and melting point, and increased the coefficient of thermal expansion and electrical resistivity. The obtained density, surface tension, and viscosity for SnZn + Ga were lower compared to the literature data for eutectic PbSn.[12,13] Increasing addition of Ga to eutectic SnZn alloys caused increasing tensile strength but reduced elongation values. The DSC measurement for the alloy SnZn + 3.0Ga (wt pct) shows dissolution, which starts around 323 K (50 °C), which is also apparent in regard to thermal expansion and electrical resistivity, and which is in line with the phase diagram of Ga-Sn-Zn.[2] The microstructures of SnZn + Ga are similar to eutectic SnZn and the same as obtained in References 4 and 15.
References
L. Zhang, K.N. Tu, Structure and properties of lead-free solders bearing micro and nano particles, Mater. Scie. Engin. R 82 (2014) 1–32
Y. Zhang, T. Liang, M.A. Jusheng, Phase diagram calculation on Sn–Zn–Ga solders, J. of Non-Cryst. Solids 336 (2004) 153–156
W-X. Chen, S-B. Xue, H. Wang, Wetting properties and interfacial microstructures of Sn-Zn-xGa solders on Cu substrate, Mater.Design. 31 (2010) 2196–2200
J.M. Song, N.S. Liu, K-L. Lin, Microstructures, Thermal and Tensile Properties of Sn-Zn-Ga Alloys, Mater. Trans. 45, (2004) 776-782
H. Ye, S. Xue, J. Luo, Y. Li, Properties and interfacial microstructure of Sn–Zn–Ga solder joint with rare earth Pr addition, Mater. Design. 46 (2013) 816–823
T. Gancarz and J. Pstrus: Arch. Metall. Mater. 2015, vol. 60, pp. 1603–07
T. Gancarz, W. Gasior, H. Henein, The Discharge Crucible Method for Making Measurements of the Physical Properties of Melts: An Overview, Int. J. Thermophys. 35 (2014) 1725–1748
T. Gancarz, W. Gasior, H. Henein, Physicochemical Properties of Sb, Sn, Zn, and Sb–Sn System, Int. J. Thermophys. 34 (2013) 250-266
W.R. Gale and T.C. Totemaier, eds.: Smithells Metals Reference Book, Elsevier, Butterworth-Heinemann Ltd., Oxford, 2004.
T. Gancarz, J. Pstruś, W. Gąsior, H.Henein, Physicochemical properties of SnZn and SAC + Bi alloys, J. of Electron. Mater. 42 (2013) 288-293
R. Novakovic, D. Zivkovic, Thermodynamics and surface properties of liquid Ga-X (X = Sn, Zn) alloys, J. of Mater. Sci. 40 (2005) 2251–2257
W. Gasior, Z. Moser, J. Pstrus, Density and surface tension of the Pb-Sn liquid alloys, J. Phase Equilib. Diff. 22 (2001) 20-25
W. Gasior, Z. Moser, J. Pstrus, and M. Kucharski, Viscosity of the lead-tin liquid alloys, Arch. Metall. Mater. 46 (2001) 23-32
SGTE—SGTE alloy phase diagrams, http://www.crct.polymtl.ca/FACT/documentation/SGTE/SGTE_Figs.htm
K-I. Chen,S-C. Cheng, S. Wu, K-L. Lin, Effects of small additions of Ag, Al, and Ga on the structure and properties of the Sn–9Zn eutectic alloy, J. Alloys. Comp. 416 (2006) 98–105
T. Gancarz, P. Fima, J. Pstruś, Thermal Expansion, Electrical Resistivity, and Spreading Area of Sn-Zn-In Alloys, J. Mater. Eng. Perform. 5 (2014) 1524-1529
W. Osorio, L. Peixoto, L. Garcia, N. Mangelinck-Noël, A. Garcia, Microstructure and mechanical properties of Sn–Bi, Sn–Ag and Sn–Zn lead-free solder alloys, J. Alloy Compd. 572 (2013) 97-106
Acknowledgment
This work was financed by the National Science Centre Poland grant 2013/09/D/ST8/03991 “Physicochemical properties of SnZn + X (X = Ga, Na) alloys” in the year 2014 to 2017.
Author information
Authors and Affiliations
Corresponding author
Additional information
Manuscript submitted April 4, 2015.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Gancarz, T. Physical, Thermal, Mechanical Properties, and Microstructural Characterization of Sn-9Zn-XGa Alloys. Metall Mater Trans A 47, 326–333 (2016). https://doi.org/10.1007/s11661-015-3235-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11661-015-3235-3