Abstract
Objective
To investigate the antiproliferative activity of Salvia miltiorrhiza Bunge. (SM) on the castration-resistant prostate cancer (CRPC) cell line DU-145, in vitro and in vivo.
Methods
Prostate cancer cell line (DU-145) and normal prostate cell line (RWPE-1) were treated with SM at different concentrations (3.125, 12.5, 25 and 50 μg/mL) to investigate the antiproliferative effects. DNA laddering analysis was performed to investigate the apoptosis of DU-145 cells. Molecular mechanism was investigated by Western blot analysis of p53, Bcl-2, prostate specific antigen (PSA), and androgen receptor (AR). Six-week-old male BALB/c nude mice were randomly divided into normal control group (n=101) and treated group (n=101) which administered 500 mg/kg SM for 2 weeks. Tumor volumes were measured.
Results
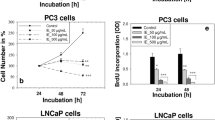
Treatment with SM resulted in a dose-dependent decrease in cell number of DU-145 cells in comparison with RWPE-1. DNA laddering analysis indicated the apoptosis of DU-145 cells. Treatment with SM increased the expression of p53 and reduced the expression of Bcl-2 proteins. The levels of PSA were considerably reduced in SM-treated group compared to the controls, and a decrease in AR expression was observed when cells were treated with SM in the same pattern as a reduction in PSA. In the tumour xenograft study, SM given once a day for 2 weeks significantly inhibited tumour growth.
Conclusion
SM might contribute to the anticancer actions such as induction of apoptosis and inhibition of proliferation of prostate cancer cells.
Similar content being viewed by others
References
Grönberg H. Prostate cancer epidemiology. Lancet 2003;361:859–864.
Kimura T, Egawa S. Epidemiology of prostate cancer in Asian countries. Int J Urol 2018;25:524–531.
Park SK, Sakoda LC, Kang D, Chokkalingam AP, Lee E, Shin HR, et al. Rising prostate cancer rates in South Korea. Prostate 2006;66:1285–1291.
Von Löw EC, Perabo FG, Siener R, Müller SC. Review. Facts and fiction of phytotherapy for prostate cancer: a critical assessment of preclinical and clinical data. In Vivo 2007;21:189–204.
Kim SJ, Kim SI. Current treatment strategies for castrationresistant prostate cancer. Korean J Urol 2011;52:157–165.
Mottet N, Bellmunt J, Bolla M, Joniau S, Mason M, Matveev V, et al. EAU guidelines on prostate cancer. Part II: Treatment of advanced, relapsing, and castration-resistant prostate cancer. Eur Urol 2011;59:572–583.
Min K, Chung JW, Ha YS, Lee JN, Kim BS, Kim HT, et al. Efficacy of androgen deprivation therapy in patients with metastatic castration-resistant prostate cancer receiving docetaxel-based chemotherapy. World J Mens Health 2020;38:226–3235.
Abate-Shen C, Shen MM. Molecular genetics of prostate cancer. Genes Dev 2000;14:2410–2434.
Cohen JH, Kristal AR, Stanford JL. Fruit and vegetable intakes and prostate cancer risk. J Natl Cancer Inst 2000;92:61–68.
Liu E, Wu J, Cao W, Zhang J, Liu W, Jiang X, et al. Curcumin induces G2/M cell cycle arrest in a p53-dependent manner and upregulates ING4 expression in human glioma. J Neurooncol 2007;85:263–270.
Gallo D, Zannoni GF, De Stefano I, Mosca M, Ferlini C, Mantuano E, et al. Soy phytochemicals decrease nonsmall cell lung cancer growth in female athymic mice. J Nutr 2008;138:1360–1364.
Zhou L, Zuo Z, Chow MS. Danshen: an overview of its chemistry, pharmacology, pharmacokinetics, and clinical use. J Clin Pharmacol 2005;45:1345–1359.
Cheng TO. Cardiovascular effects of Danshen. Int J Cardiol 2007;121:9–22.
Wang X, Morris-Natschke SL, Lee KH. New developments in the chemistry and biology of the bioactive constituents of Tanshen. Med Res Rev 2007;27:133–148.
Alessandri G, Filippeschi S, Sinibaldi P, Mornet F, Passera P, Spreafico F, et al. Influence of gangliosides on primary and metastatic neoplastic growth in human and murine cells. Cancer Res 1987;47:4243–4247.
Liu JJ, Liu WD, Yang HZ, Zhang Y, Fang ZG, Liu PQ, et al. Inactivation of PI3k/Akt signaling pathway and activation of caspase-3 are involved in tanshinone I-induced apoptosis in myeloid leukemia cells in vitro. Ann Hematol 2010;89:1089–1097.
Lee CY, Sher HF, Chen HW, Liu CC, Chen CH, Lin CS, et al. Anticancer effects of tanshinone I in human non-small cell lung cancer. Mol Cancer Ther 2008;7:3527–3538.
Suh SJ, Jin UH, Choi HJ, Chang HW, Son JK, Lee SH, et al. Cryptotanshinone from Salvia miltiorrhiza Bunge has an inhibitory effect on TNF-alpha-induced matrix metalloproteinase-9 production and HASMC migration via down-regulated NF-kappaB and AP-1. Biochem Pharmacol 2006;72:1680–1689.
Wang HG, Rapp UR, Reed JC. Bcl-2 targets the protein kinase Raf-1 to mitochondria. Cell 1996;87:629–638.
Tilley WD, Buchanan G, Hickey TE, Bentel JM. Mutations in the androgen receptor gene are associated with progression of human prostate cancer to androgen independence. Clin Cancer Res 1996;2:277–285.
Linja MJ, Savinainen KJ, Saramäki OR, Tammela TL, Vessella RL, Visakorpi T. Amplification and overexpression of androgen receptor gene in hormone-refractory prostate cancer. Cancer Res 2001;61:3550–3555.
Gregory CW, He B, Johnson RT, Ford OH, Mohler JL, French FS, et al. A mechanism for androgen receptormediated prostate cancer recurrence after androgen deprivation therapy. Cancer Res 2001;61:4315–4319.
Fujita K, Nonomura N. Role of androgen receptor in prostate cancer: a review. World J Mens Health 2019;37:288–295.
Park SW, Kim JH, Lee HJ, Shin DH, Lee SD, Yoon S. The expression of androgen receptor and its variants in human prostate cancer tissue according to disease status, and its prognostic significance. World J Mens Health 2019;37:68–77.
Pienta KJ, Bradley D. Mechanisms underlying the development of androgen-independent prostate cancer. Clin Cancer Res 2006;12:1665–1671.
Tran C, Ouk S, Clegg NJ, Chen Y, Watson PA, Arora V, et al. Development of a second-generation antiandrogen for treatment of advanced prostate cancer. Science 2009;324:787–790.
Lippman SM, Klein EA, Goodman PJ, Lucia MS, Thompson IM, Ford LG, et al. Effect of selenium and vitamin E on risk of prostate cancer and other cancers: the Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA 2009;301:39–51.
Thompson IM, Goodman PJ, Tangen CM, Lucia MS, Miller GJ, Ford LG, et al. The influence of finasteride on the development of prostate cancer. N Engl J Med 2003;349:215–224.
Gomella LG. Chemoprevention using dutasteride: the REDUCE trial. Curr Opin Urol 2005;15:29–32.
Author information
Authors and Affiliations
Contributions
WJB drafted the manuscript, and participated in every part of the experiments. USH participated in the design of the study and helped in the experiments and drafting of the manuscript. KSK and JBC participated in the design, biochemical assays and statistical analysis of the study. SHH participated in the experiments, coordinated among the authors and helped to draft the manuscript. JYL, TKH, WZP and SYH participated in the design of the study and drafting of the manuscript. SWK participated in the design of the study and experiments of the study.
Corresponding author
Ethics declarations
The authors have nothing to disclose.
Additional information
Supported by the Traditional Korean Medicine Project, Republic of Korea (No. F110004), and the Next-Generation BioGreen 21 Program (No. PJ011290012016), Rural Development Administration, Republic of Korea
Rights and permissions
About this article
Cite this article
Bae, W.J., Choi, J.B., Kim, K.S. et al. Inhibition of Proliferation of Prostate Cancer Cell Line DU-145 in vitro and in vivo Using Salvia miltiorrhiza Bunge.. Chin. J. Integr. Med. 26, 533–538 (2020). https://doi.org/10.1007/s11655-017-2801-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11655-017-2801-5