Abstract
Objective
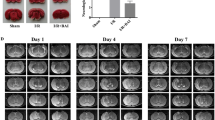
To investigate the neuro-protective effects of baicalin in Wistar rats with focal cerebral ischemic reperfusion injury.
Methods
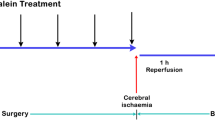
Ninety adult male Wistar rats weighing 320–350 g were randomly divided into the following groups (n=5): (a) sham control group; (b) vehicle group, subjected to middle cerebral artery occlusion and received vehicle intraperitoneally; (c-e) baicalin groups, which were subjected to the middle cerebral artery occlusion and treated with baicalin 25, 50 and 100 mg/kg, respectively. The neurological scores were determined at postoperative 1, 3 and 7 d after the treatment. The expression of protease-activated receptor-1 (PAR-1), PAR-1 mRNA and Caspase-3 were determined using Western blot, reverse transcription polymerase chain reaction (RTPCR) analysis and immunohistochemistry, respectively.
Results
Significant decrease was noted in the neurological score in the baicalin group compared with that of the vehicle group (P<0.01). Additionally, down-regulation of PAR-1 mRNA, PAR-1 and Caspase-3 was observed in the baicalin groups compared with those obtained from the vehicle group (P<0.01). Compared with the low-dose baicalin group (25 mg/kg), remarkable decrease was noted in neurological score, and the expression of PAR-1 mRNA, PAR-1 as well as Caspase-3 in the high-dose group (P<0.05).
Conclusion
Baicalin showed neuro-protective effects in focal cerebral ischemic reperfusion injury through inhibiting the expression of PAR-1 and apoptosis.
Similar content being viewed by others
References
Hong KS, Saver JL. Quantifying the value of stroke disability outcomes: WHO global burden of disease project disability weights for each level of the modified Rankin Scale. Stroke 2009;40:3828–3833.
Miyahara S, Kiryu J, Tsujikawa A, Katsuta H, Nishijima K, Miyamoto K, et al. Argatroban attenuates leukocyte- and platelet-endothelial cell interactions after transient retinal ischemia. Stroke 2003;34:2043–2049.
Zhou QB, Shao NF, Bi JZ. Exploration on the application of heat-clearing and detoxicating in treating stroke in acute stage. Chin J Integr Tradit West Med (Chin) 2004;24:263–264.
Zhou QB, Li LY, Jia Q. Protective effect of Noningkang Granule on brain in intracerebral hemorrhagic rats. Chin J Integr Tradit West Med (Chin) 2007;27:814–818.
Li BQ, Fu T, Yan YD, Baylor NW, Ruscetti FW, Kung HF. Inhibition of HIV infection by baicalin—a flavonoid compound purified from Chinese herbal medicine. Cell Mol Biol Res 1993;39:119–124.
Jung SH, Kang KD, Ji D, Fawcett RJ, Safa R, Kamalden TA, et al. The flavonoid baicalin counteracts ischemic and oxidative insults to retinal cells and lipid peroxidation to brain membranes. Neurochem Int 2008;53:325–337.
Tian H, Zhang X, Wu C, Chen L, Ying R, Ye J, et al. Effects of Baicalin and Octreotide on the serum TNF-alpha level and apoptosis in multiple organs of rats with severe acute pancreatitis. Inflammation 2009;32:191–201.
Li-Weber M. New therapeutic aspects of flavones: the anticancer properties of Scutellaria and its main active constituents Wogonin, Baicalein and Baicalin. Cancer Treat Rev 2009;35:57–68.
Cao Y, Mao X, Sun C, Zheng P, Gao J, Wang X, et al. Baicalin attenuates global cerebral ischemia/reperfusion injury in gerbils via anti-oxidative and anti-apoptotic pathways. Brain Res Bull 2011;85:396–402.
Coughlin SR. Thrombin signalling and protease-activated receptors. Nature 2000;407:258–264.
Mao Y, Zhang M, Tuma RF, Kunapuli SP. Deficiency of PAR4 attenuates cerebral ischemia/reperfusion injury in mice. J Cereb Blood Flow Metab 2010;30:1044–1052.
Striggow F, Riek M, Breder J, Henrich-Noack P, Reymann KG, Reiser G. The protease thrombin is an endogenous mediator of hippocampal neuroprotection against ischemia at low concentrations but causes degeneration at high concentrations. Proc Natl Acad Sci USA 2000;97:2264–2269.
Vaughan PJ, Pike CJ, Cotman CW, Cunningham DD. Thrombin receptor activation protects neurons and astrocytes from cell death produced by environmental insults. J Neurosci 1995;15:5389–5401.
Choi SH, Joe EH, Kim SU, Jin BK. Thrombin-induced microglial activation produces degeneration of nigral dopaminergic neurons in vivo. J Neurosci 2003;23:5877–5886.
Gingrich MB, Traynelis SF. Serine proteases and brain damage—is there a link? Trends Neurosci 2000;23:399–407.
Wang H, Ubl JJ, Stricker R, Reiser G. Thrombin (PAR-1)-induced proliferation in astrocytes via MAPK involves multiple signaling pathways. Am J Physiol Cell Physiol 2002;283:C1351–C1364.
Junge CE, Sugawara T, Mannaioni G, Alagarsamy S, Conn PJ, Brat DJ, et al. The contribution of protease-activated receptor 1 to neuronal damage caused by transient focal cerebral ischemia. Proc Natl Acad Sci USA 2003;100:13019–13024.
Sokolova E, Reiser G. Prothrombin/thrombin and the thrombin receptors PAR-1 and PAR-4 in the brain: localization, expression and participation in neurodegenerative diseases. Thromb Haemost 2008;100:576–581.
Olson EE, Lyuboslavsky P, Traynelis SF, McKeon RJ. PAR-1 deficiency protects against neuronal damage and neurologic deficits after unilateral cerebral hypoxia/ ischemia. J Cereb Blood Flow Metab 2004;24:964–971.
Koizumi J, Yoshida Y, Nakazawa T, Ooneda G. Experimental studies of ischemic brain edema.1:a new experimental model of cerebral embolism infarcts in the ischemic area. Jpn J Stroke 1986;8:1–8.
Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke 1989;20:84–91.
Berge E, Friis P, Sandset PM. Hemostatic activation in acute ischemic stroke. Thromb Res 2001;101:13–21.
McConnell JP, Cheryk LA, Durocher A, Bruno A, Bang NU, Fleck JD, et al. Urinary 11-dehydro-thromboxane B(2) and coagulation activation markers measured within 24 h of human acute ischemic stroke. Neurosci Lett 2001;313:88–92.
Hua Y, Wu J, Keep RF, Hoff JT, Xi G. Thrombin exacerbates brain edema in focal cerebral ischemia. Acta Neurochir Suppl 2003;86:163–166.
Luo W, Wang Y, Reiser G. Protease-activated receptors in the brain: receptor expression, activation, and functions in neurodegeneration and neuroprotection. Brain Res Rev 2007;56:331–345.
Li H, Wu H, Huang XY, Cong YW, Song YJ, Zhou M, et al. The expression and significance of thrombin and PAR-1 following focal cerebral ischemia reperfusion in rats. Stroke Nerv Dis 2009;1:20–22.
Gong C, Boulis N, Qian J, Turner DE, Hoff JT, Keep RF. Intracerebral hemorrhage-induced neuronal death. Neurosurgery 2001;48:875–882; discussion 882–883.
Reimann-Philipp U, Ovase R, Weigel PH, Grammas P. Mechanisms of cell death in primary cortical neurons and PC12 cells. J Neurosci Res 2001;64:654–660.
Yang WQ, Sun SG, Tong ET, Cao XB. Effect of thrombin on intracellular free calcium in primary cultured hippocampal neurons. Stroke Nerv dis 2005;21:188–192.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by National Natural Science Foundation of China (No. 81072916), Shandong Science and Technique Foundation (No. 2005GG3202062), Shandong Traditional Chinese Medicine Administration Fund Program (No.2009-160) and Free Exploration Program of Shandong University (No.2009TS009)
Rights and permissions
About this article
Cite this article
Zhou, Qb., Duan, Cz., Jia, Q. et al. Baicalin attenuates focal cerebral ischemic reperfusion injury by inhibition of protease-activated receptor-1 and apoptosis. Chin. J. Integr. Med. 20, 116–122 (2014). https://doi.org/10.1007/s11655-013-1441-7
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11655-013-1441-7