Abstract
Objective
To observe the effect of extracts from Radix Ginseng, Radix Notoginseng and Rhizoma Chuanxiong (EXT) on delaying vascular smooth muscle cells (VSMCs) aging in aged rats.
Methods
VSMCs were obtained by the modified tissue explants technique and were shown to be positive for smooth muscle α-actin (SM-α-actin) by immunohistochemistry staining. VSMCs obtained from the young rats were served as the young control group; VSMCs obtained from the old rats were treated with no drug (the old group), with low dose extracts (20 mg/L, the EXT low-concentration group) and high dose extracts (40 mg/L, the EXT highconcentration group), and with Probucal (10−6 mol/L, the Probucal group) as a positive control. All groups were cultured for 24 h in the medium with 10% serum for 24 h followed by another 24 h in the serum-free medium. At the end of the 48-h culture, the following analyses were performed including determination of senescence-associated β-galactosidase (SAβ-Gal) activity, flow cytometry analysis of cell cycle, real-time quantitative reverse transcription polymerase chain reaction (RT-PCR) analyses of p16, Cyclin D1, cyclin-dependent kinase 4 (CDK4) and retinoblastoma (Rb) mRNA expression, and Western blotting analyses of p16, cyclin D1, CDK4 and phosphoretinoblastoma (pRb) protein expressions.
Results
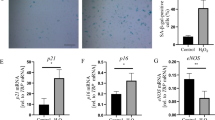
(1) In comparison to the younger rats, VSMCs from aged rats had significantly more SAβ-Gal positive cells (P<0.01) and more cells in S phase (P<0.05). VSMCs from the all treated groups showed a significant decrease in both SAβ-Gal positive cells (P<0.05) and S phase (P<0.05) compared to the old rats. (2) Compared with the young group, VSMCs in the old group had a significant decrease in p16 and Rb mRNA expression and a significant increase in Cyclin D1 and CDK4 mRNA expression. Compared with the old group, VSMCs in the treated groups had a significant increase in p16 and Rb mRNA expression and a significant decrease in Cyclin D1 and CDK4 mRNA expression (P<0.05). (3) Compared with the young group, VSMCs in the old group had a significant decrease in p16 protein expression and a significant increase in Cyclin D1, CDK4 and pRb protein expressions (P<0.05). Compared with the old group, VSMCs in the treated groups had a significant increase in p16 protein expression and a significant decrease in cyclinD1, CDK4 and pRb protein expressions (P<0.05).
Conclusions
VSMCs obtained from old rats showed typical signs of cellular senescence and vascular aging. EXT had an effect on delaying senescence of VSMCs in vitro by altering the p16-cyclinD/CDK-Rb pathway.
Similar content being viewed by others
References
Orlandi A, Bochaton-Piallat ML, Gabbiani G. Aging, smooth muscle cells and vascular pathobiology: implications for atherosclerosis. Atherosclerosis 2006;188:221–230.
Min LJ, Mogi M, Iwanami J, Li JM, Sakata A, Fujita T, et al. Cross-talk between aldosterone and angiotensin II in vascular smooth muscle cell senescence. Cardiovasc Res 2007;76:506–516.
Huang YL, Lu T. Ageing and endothelial dysfunction. Adv Cardiovasc Dis (Chin) 2007;9:766–770.
Lakatta, EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: part I: aging arteries: a ’set up’ for vascular disease. Circulation 2003;107:139–146.
Yang J, Lei Y. The research development and relationship of celluar senescence and vascular aging. Chin J Integr Tradit West Med (Chin) 2009;29:369–374.
Li Z, Cheng H, Lederer WJ, Froehlich J, Lakatta EG. Enhanced proliferation and migration and altered cytoskeletal proteins in early passage smooth muscle cells from young and old rat aortic explants. Exp Mol Pathol 1997;64:1–11.
Wang M, Monticone RE, Lakatta EG. Aterial aging: a journey into subclinical arterial disease. Pathophysiology of hypertension. Curr Opin Nephrol Hypertens 2010;19:201–207.
Li H, Bai XJ, Chen XM. Progress on the study of mechanism and biomarkers of arterial and cardiac aging. Natl Med J China (Chin) 2005;85:212–215.
Gorenne I, Kavurma M, Scott S, Bennett M. Vascular smooth muscle cell senescence in atherosclerosis. Cardiovasc Res 2006;72:9–17.
Burrig KF. The endothelium of advanced arteriosclerotic plaques in humans. Arterioscler Thromb 1991;11:1678–1689.
Bennett MR, Macdonald K, Chan SW, Boyle JJ, Weissberg PL. Cooperative interactions between Rb and p53 regulate cell proliferation, cell senescence, and apoptosis in human vascular smooth muscle cells from atherosclerotic plaques. Circ Res 1998;82:704–712.
Yang D, McCrann DJ, Nguyen H, St Hilaire C, DePinho RA, Jones MR, et al. Increased polyploidy in aortic vascular smooth muscle cells during aging is marked by cellular senescence. Aging Cell 2007;6:257–260.
McCrann DJ, Yang D, Chen H, Carroll S, Ravid K. Upregulating of Nox4 in the aging vasculature and its association with smooth muscle polyploidy. Cell Cycle 2009;8:902–908.
Minamino T, Miyauchi H, Yoshida T, Ishida Y, Yoshida H, Komuro I. Endothelial cell senescence in human atherosclerosis: role of telomere in endothelial dysfunction. Circulation 2002;105:1541–1544.
Stemerman MB, Weinstein R, Rowe JW, Maciag T, Fuhro R, Gardner R. Vascular smooth muscle cell growth kinetics in vivo in aged rats. Proc Natl Acad Sci U S A 1982;79:3863–3866.
Yang YF. The effect of tonifying qi and activating blood in prevention and treatment cardiovascular disease. J Clin Exp Med (Chin) 2007;6(8):138–139.
Zhang JL, Xu WA, Yang YK, Wu SF, Xian HJ, Wen ZY. Effect of the panaxnotoginsen Rb1 on lipid peroxidetion of aged mouse. Acad J Kunming Med Coll (Chin) 2001;22:45–46.
Zhou J. Study on effective ingredients of Ligusticum Chuanxiong Hort and its pharmacological action. J Zhejiang Tradit Med (Chin) 2007;42:615–616.
Shi JM, Luo LF. The clinical observation of treating 30 cases of coronary disease angina pectoris by reinforcing qi activating blood therapeutics. China Med Herald (Chin) 2007;4(9):103–104.
Huang HS, Tan YZ, Tang YM, Zhang TY, Cai MH. Experimental study on protecting effects of Yiqi Huoxue Recipe in myocardial ischemic models in dogs. Chin J Integr Tradit West Med (Chin) 1996;16 (Suppl):6–8.
Gao SW, Wang WH, Qin LM, Niu FL, Wang SR, Lu JQ, et al. Effects of some Chinese herbs on changes of energy and lipid peroxidation in cardiac cells induced by angiotensin II. J Beijing Univ Tradit Chin Med (Chin) 2005;28(6):32–35.
Gao YY, Liu WL, Zhou BR, Zhou BR, Li W, Luo D. Ginsenoside-Rg1 protects human dermal fibroblasts against 8-methoxypsoralen plus ultraviolet A-induced photoaging through p53-dependent signaling pathways. Chin Pharmacol Bull 2010;26:383–387.
Lei Y, Yang J, Zhao H, Du XJ, Liu JG, Chen KJ, et al. Experimental study on extracts from Ginseng, Notoginseng and Chuanxiong for delaying vascular aging in senescent mice. Chin J Integr Tradit West Med (Chin) 2010;30:946–951.
Lu MH, Wu GC, Chao CF. Comparison of endothelin binding sites in cultured aortic smooth muscle cells from spontaneously hypertensive and nomotensive rats. Jap Circ J 1997;61:145–150.
Endlich N, Endlich K, Taesch N, Helwig JJ. Culture of vascular smooth muscle cells from small arteries of the rat kidney. Kidney Int 2000;57:2468–2475.
Chamley-Campbell J, Cambell GR, Ross R. The smooth muscle cell in culture. Physiol Rev 1979;59:1–61.
Gorenne I, Kavurma M, Scott S, Bennett M. Vascular smooth muscle cell senescence in atherosclerosis. Cardiovasc Res 2006;72:9–17.
Minamino T, Komuro I. Vascular cell senescence: contribution to atherosclerosis. Circ Res 2007;100:15–26.
Dimri GP, Lee X, Basile G, Acosta M, Scott G, Roskelley C, et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci 1995;92:9363–9367.
Minamino T, Miyauchi H, Yoshida T, Tateno K, Kunieda T, Komuro I. Vascular cell senescence and vascular aging. J Mol Cell Cardiol 2004;36:175–183.
Ruan YJ, Yin MZ, Wu SZ. The research on vascular aging. Pract Senior Med (Chin) 2006;20:418–420.
Yildiz O. Vascular smooth muscle and endothelial functions in aging. Ann New York Acad Sci 2007;1100:353–360.
Zhou G, Fan CW. Antioxidant and anti-aging. Chin J Hydropower Med (Chin) 2008;1:34–35.
Li Y, Feng X, Wang XY. Protective effect of probucol on the kidney of aging rats induced by D-galactose. J Chongqing Med Univ (Chin) 2011;36:171–173.
Cheng XX, Wang J. Clinical application of the antioxidant probucol. Chin J Clin Ration Drug Use (Chin) 2009;13:125–126.
Sherr CJ. G1 phase progression: cycling on cue. Cell 1994;79:551–555.
Lee JY, Bielawska AE, Obeid LM. Regulation of cyclindependent kinase 2 activity by ceramide. Exp Cell Res 2000;261:303–311.
Sandhu C, Peehl DM, Slingerland J. p16INK4A mediates cyclin dependent kinase 4 and 6 inhibition in senescent prostatic epithelial cells. Cancer Res 2000;60:2616–2622.
Thomas NS. Thomas, Pizzey AR, Tiwari S, Williams CD, Yang J. p130, p107, and pRb are differentially regulated in proliferating cells and during cell cycle arrest by α-interferon. J Biol Chem 1998;37:23659–23667.
Rodriguez-Menocal L, Pham SM, Mateu D, St-Pierre M, Wei Y, Pestana I, et al. Aging increase p16INK4a expression in vascular smooth-muscle cells. Biosci Rep 2010;30:11–18.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by the National Basic Research Program of China (Program 937, No. 2007CB507400), the National Natural Science Foundation of China (No. 30973828), the Independent Topic Program of China Academy of Chinese Medical Sciences (No. Z02151)
Rights and permissions
About this article
Cite this article
Tao, Ll., Lei, Y., Wang, Gl. et al. Effect of extracts from Radix Ginseng, Radix Notoginseng and Rhizoma Chuanxiong on delaying aging of vascular smooth muscle cells in aged rats. Chin. J. Integr. Med. 18, 582–590 (2012). https://doi.org/10.1007/s11655-012-1180-1
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11655-012-1180-1