Abstract
Obesity is one of the principal reasons behind a wide range of metabolic diseases and dramatic health complications. Recent studies shed the light on chia (Salvia hispanica L., Lamiaceae) and quinoa (Chenopodium quinoa Willd., Amaranthaceae) seeds and identified them as products of utmost health benefits. The present study was designed to explore the molecular mechanisms of the hydroalcoholic extract of those seeds in weight management. Rats were divided randomly into two main groups: control and treated. The control groups received regular chow diet, high-fat diet, and green tea. The treated groups received high-fat diet and chia or quinoa extracts. Results revealed that the seeds showed hepatoprotective effects and anti-inflammatory and antioxidant activities, and modulated leptin, adiponectin, serum lipid, and glycemic profiles. Effects are further consolidated by normal hepatic tissue architecture upon histopathological examination. Moreover, modulation of peroxisome proliferator-activated receptors-γ transcriptional activity via activation of 5′AMP-activated protein kinase and suppression of nuclear expression of sterol regulatory element-binding protein-1c in obese rats as compared to green tea were demonstrated. Characterization of the major secondary bioactive metabolites was done using HPLC/PDA/ESI-MS/MS. Our study advocates evidence-based study on nutrition and health claims on the use of chia and quinoa extracts as nutraceutical supplements for promoting weight wellness and alleviating its related metabolic disorders.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obesity and metabolic syndrome represent chief universal public health challenges. Healthcare professionals can revisit it as “the world’s new syndrome.” We as humans have evolved effective physiological mechanisms to protect against weight loss, but failed to be protected against weight gain in times of food abundance (Hill and Peters 1998). Moreover, due to sedentary lifestyle and risky behaviors such as unhealthy eating, smoking, and excessive alcohol, the world is confronted more than ever with the noncommunicable diseases (NCDs), including heart disease, stroke, cancer, diabetes, and chronic lung disease (Emanuela et al. 2012). Additionally, several studies suggested the link between metabolic syndrome and the etiology of certain disease such as cancer (Rasheed et al. 2022) and cognitive impairments manifested in Alzheimer’s disease (Vanhanen et al. 2006). The diagnosis of metabolic syndrome is confirmed when patients meet at least three of the following five criteria: hypertension, abdominal obesity, high triacylglyceride (TG), low high-density lipoprotein-cholesterol (HDL-C), and insulin resistance (IR) related to type 2 diabetes mellitus (T2DM) (Ascaso et al. 2003). Noteworthy, reducing body weight by 5–10% greatly lowers metabolic syndrome components, and hence the risk of fatal comorbidities (Magkos et al. 2016).
Recently, the introduction of healthy lifestyle has gained wide popularity against the battle of many chronic diseases including obesity and related disorders. Unfortunately, dieting and exercising fail in some individuals. Pharmacological treatment should only be advised in case of body mass index (BMI) > 30 or BMI ≥ 27 with comorbidity (Apovian et al. 2015). Consequently, synthetic drugs, especially appetite suppressors acting on the CNS and bariatric surgeries, in many instances, result in several complications (Fassina et al. 2015). Henceforth, safe pharmacological interventions become dictated.
Natural products incorporated in either the foodstuffs or pharmaceutical products are offering an alternative approach for obesity management with fewer side effects (Kazemipoor et al. 2012). Those are mostly of plant origin, including seeds, grains, vegetables, fruits, and herbs. The biological effects of these natural products are attributed to the abundance of active redox chemicals (antioxidants), fibers, and unsaturated fatty acids. Noteworthy, grape seed extract (Liu et al. 2020a, b), fenugreek seeds (Yao et al. 2020), and green coffee beans (Murase et al. 2011) are widely used in weight management. Endowed with anti-inflammatory, antioxidant, lipid profile adjustment, and antidiabetic effects, chia, Salvia hispanica L., Lamiaceae (Scapin et al. 2016), and quinoa, Chenopodium quinoa Willd., Amaranthaceae (Noratto et al. 2019), are medicinal plants of high nutritive value, ascribed with many health benefits (Kulczyński et al. 2019). The seeds are a rich source of fibers, polyphenols, and omega-3 fatty acids that reduce inflammation and oxidative stress, and enhance cognitive functions (De Falco et al. 2017; Liu et al. 2020a, b).
The peroxisome proliferator-activated receptors (PPARs) are transcription factors that modulate a wide array of physiological processes comprising cell growth, differentiation, membrane lipid synthesis/turnover, and homeostasis (Ahmed et al. 2007), highly expressed in muscles (Barish et al. 2006). Moreover, these receptors regulate the expression of genes involved in fatty acid metabolism and obesity (Ahmed et al. 2007). AMP-activated protein kinase (AMPK) is an imperative energy sensor in cells (Chanda et al. 2009) and its activation stimulates fatty acid oxidation in liver and heart, inhibits hepatic lipogenesis and adipocyte differentiation, and enhances glucose uptake by muscles (Lage et al. 2008). Growing body of evidence suggests that PPAR-γ agonists modulate the activity of (AMPK), probably via SIRT 1 (a protein that plays a key role in hepatic lipid metabolism) activation (Chanda et al. 2009).
The present study was designed to investigate the potential therapeutic merits of chia and quinoa seeds in weight loss and comparing their effects with green tea as a reference herb. Mechanistic insights into the AMPK-α modulating PPAR-ɣ pathway regulating lipogenesis, adipogenesis, and inflammation have also been explored on the phenolic profiles of both plants.
Materials and Methods
Plant Material
Fresh seeds (1 kg) of chia, Salvia hispanica L., Lamiaceae, and quinoa seeds, Chenopodium quinoa Willd., Amaranthaceae, were obtained from Agricultural Genetic Engineering Research Institute, Cairo, Egypt, in April 2018. Taxonomist Dr. Mahmoud Zidan kindly authenticated the seeds. Specimens of the identified seeds were deposited at the herbarium of the Department of Pharmaceutical Biology, Faculty of Pharmacy and Biotechnology, German University in Cairo (Chia: GUCPHBL1 2019; Quinoa: GUCPHBL2 2019).
Initially, defatting of seeds was done with hexane (Sigma Aldrich, Munich, Germany) followed by exhausting with 75% ethanol-water until complete extraction. The supernatant was filtered on #4 Whatman filters and then evaporated under reduced pressure at temp. 40 °C using a rotavapor (Buchi, R-210, Switzerland). They yielded semisolids of 6.5 w/w% for chia and 9.5 w/w% for quinoa. Similarly, hydroalcoholic extract was obtained from 2 kg of green tea leaves. The amount of total phenolics was determined quantitatively using Folin-Ciocalteau’s reagent and expressed as gallic acid equivalent per gram of dry weight of the plant extract (mg GAE/g DW). Flavonoids were measured in milligram per gram quercetin equivalent per gram of dry weight of the extract using the previously mentioned adapted aluminum chloride, Merck (Darmstadt, Germany) colorimetric assay (Aryal et al. 2019).
Animals
Adult male white albino Wistar rats, weighing 150–200 g, were obtained from the National Research Centre (Cairo, Egypt). They were housed in standard polypropylene cages, where each cage contained 6 rats and were housed under appropriate conditions of temperature (25 ± 2 °C), humidity (60–70%), and light (12-h dark/light cycles). The animals were left for 1 week before experimental intervention for acclimatization at the GUC animal house. Free access to food and water was allowed, prior to the dietary manipulation.
Experimental Design
Based on our preliminary study, dose selection was conducted, where the optimum doses for chia and quinoa hydroalcoholic extracts were selected for statistical analysis fulfilling safety criteria and weight loss results. Accordingly, chia dose of 200 mg/kg b.w./day and quinoa dose of 200 mg/kg b.w./day were selected. As shown in Fig. S1, animals were divided into five groups (n = 6/group) along the 6 weeks.
-
Group I: normal food diet (NFD) were fed normal food (chow) diet and saline.
-
Group II: high-fat diet (HFD) animals were fed high fat diet and saline.
-
Group III: (HFD + green tea) animals on HFD, received green tea extract in dose 250 mg/kg/day.
-
Group IV: (HFD + quinoa) animals on HFD, received quinoa in dose 250 mg/kg/day dissolved in saline (Ghahremanloo et al. 2018), and
-
Group V: (HFD + chia) animals on HFD, received chia in dose 250 mg/kg/day dissolved in saline (Apoorva et al. 2020).
All groups received treatments orally. Animals were fasted overnight and anesthetized with diethyl ether (Camlab, UK). Twenty-four hours after the last dose, rats were sacrificed, and blood samples were collected from the animal’s venous sinus using capillary tubes (Shalaby et al. 2014) and centrifuged using (Centurion, Scientific Ltd., USA) at 373 × g for 20 min. Sera were collected and stored at − 80 °C for further biochemical analysis. Moreover, liver and visceral fats were excised, cleaned, weighted, and recorded for each group. Liver adipose tissues were homogenized and stored in − 80 °C for western blotting of PPAR-ɣ and AMPK-1α expression, and for PCR analysis of gene expression for free fatty acids (FFAs) and sterol regulatory element-binding protein 1 (SREBP-1C) expression. Moreover, the liver adipose tissues were collected in 10% formalin for histopathological investigations. In this experiment, rats were randomly assigned according to a randomization table (Shalaby et al. 2014). A technical assistant performed animals’ allocation and drug treatment in a blinded manner. All biochemical and histopathological examinations were assessed by investigators blinded to the experimental groups.
Biochemical Parameters
The following parameters were assessed in animal sera colorimetrically using their corresponding kits. Alanine transaminase (ALT) and aspartate transaminase (AST) (BioMed-GPT, and BioMed-GOT, Cairo, Egypt, respectively). Total cholesterol (TC), triacylglycerides (TG), high-density lipoprotein (HDL) by Spectrum Egyptian Co. for Biotechnology, Cairo, Egypt. Low-density lipoprotein (LDL) (Stanbio laboratory, Boerne, TX, USA), fasting blood glucose (FBG) (BioMed-glucose L.S, Egy-chem, Cairo, Egypt), malondialdehyde (MDA) and glutathione (GSH) (Biodiagnostic, Giza, Egypt).
Chromatographic Analysis
The analysis was performed using Waters Corp. (USA) delivery system accessorized with an ACQUITY H-Class-Xevo TQD triple-quadruple tandem mass spectrometer with an electrospray ionization (ESI) interface. Methanol was used to dissolve the samples (Sigma Aldrich, Germany), in a concentration of 1 mg/ml, followed by filtration using a micropore filter of 0.2-μm pore size. The mobile phase composition was 0.01% formic acid in purified water (A), and acetonitrile (B) with the following elution gradient: 0–4 min, 15% B; 4–5 min, 20% B; 5–10 min, 30% B; 10–11 min, 45% B; 11–33 min, 55% B; 33–35 min, 70% B; 35–40 min, 90% B; 40–45 min, 15% B. The injection volume was 10 μl and the flow rate was 1 ml/min. At mass ranges (100–1000 m/z), the analytics were observed. MS scans were performed at 3.5 kV capillary voltage, 20–95 V cone voltage detection, 2.5 V radio frequency (RF) lens voltage, 150 °C source temperature, and 500 °C desolvation gas temperature. At flow rates of 1000 and 20 l/h, nitrogen was used as a desolvation and cone gas, respectively. Mass Lynx 4.1 software was used to monitor system operation and data collection (Elshamy et al. 2021).
Antioxidant Assay
The antioxidant effects of the different samples were measured using 2,2-diphenyl1-picrylhydrazyl (DPPH•) radical scavenging assay, according to (Scapin et al. 2016), and the cupric ion reducing activity (CUPRAC) was determined according to method described by Apak et al. (2006).
Enzyme Link Immunosorbent Assay
Commercial ELISA kits were used, according to the manufacturer’s instructions, to measure serum insulin (Cusabio, Wuhan, China) and liver homogenate leukotriene LT-B4, leptin, and adiponectin (MyBioSource, San Diego, USA, Cusabio, Kampenhout, Belgium and RayBiotech, Inc., Norcross, GA, USA). Results were expressed as picogram/milligram of protein.
Quantitative Real-time PCR
The expressions of FFAs and SREBP-1C genes were determined using RT-PCR. Total RNA was extracted from liver tissues using RNeasy RNA extraction Kit (Qiagen, MD, USA). First, the purity of extracted RNA was verified using spectrophotometry (dual-wavelength Beckman, Spectrophotometer, USA) by recording the optical density (OD) at 260/280 nm. Second, equal amounts of extracted RNA were reverse-transcribed using RT-PCR kit (Fermentas, Waltham, USA). Third, quantitative RT-PCR was carried out using SYBR Green. The sequences of primers used in the present investigation are presented in Table S1.
Western Blot Analysis
Liver tissue levels of PPAR-ɣ and AMPK-1α were analyzed using the western blot methodology. Liver homogenate was prepared in RIPA buffer and centrifuged, and protein concentration was determined using Bradford Protein Assay Kit (Bio Basic Inc., Markham, Canada) for quantitative protein analysis. Equal amounts of protein solutions were separated using SDS-PAGE (10% acrylamide gel) (Bio-Rad Laboratories, California, USA). The separated proteins were transferred onto polyvinylidene difluoride (PVDF) membranes and blocked with tris-buffered saline and tween 20 (TBST) buffer and 3% bovine serum albumin (BSA) for 1 h at room temp. Next, the membranes were incubated overnight at 4 °C with the primary antibodies against PPAR-ɣ and AMPK-1α (Thermo Fisher Scientific, MA, USA). After washing with TBST three times, membranes were incubated with horseradish peroxidase (HRP)-conjugated goat anti-rabbit- IgG (Novus Biologicals, CO, USA) for 1 h at room temperature. Finally, the band intensity was visualized using a ChemiDoc™ imaging system (ClarityTM Western ECL substrate, Bio-Rad, CA, USA) with Image Lab™ software version 5.1 (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Histopathological Examination
Liver tissue samples were collected and fixed in 10% buffered formalin for 24 h. Afterwards, they were processed and embedded in paraffin wax sections for further histopathological investigation using hematoxylin and eosin (H&E) stain (Sigma Aldrich St. Louis, MO, USA) (Bancroft and Gamble 1996).
Statistical Analysis
GraphPad Prism software, version 5.01, was used for the statistical analysis of the groups. Results were presented as mean ± SD (standard deviation) and assessed using the one-way analysis of variance (ANOVA) procedure followed by Tukey’s multiple range test. A p value less than 0.05 was considered statistically significant, where ap < 0.001, bp < 0.01, and cp < 0.05; ns = statistically not significant.
Results and Discussion
Total Phenolic and Flavonoid Contents
The total polyphenol and total flavonoid contents observed in quinoa were 28.2 mg gallic acid equivalent (GAE)/g and 247 mg quercetin equivalents (QE)/g; in chia were 53.2 mg gallic acid equivalent (GAE)/g) and mg quercetin equivalents (QE)/g, respectively.
Dereplication of Bioactive Metabolites
The constituents in the bioactive extracts were detected using the HPLC/PDA/ESI/MS method, as presented in Tables S1 and S2. The peaks were interpreted by comparison with reported data for the fragmentation pattern of the deprotonated ion [M − H]− (Table S2 and S3). Figure S2 illustrates the chromatograms obtained from both the chia (Fig. S2A) and quinoa (Fig. S2B), where the following major antioxidants components were dereplicated and should be mentioned: salvianolic acid C (1; Rt 12.75 min; 13.8%) from chia seeds, and rosmarinic acid (2; Rt 12.72 min; 14.9%) from quinoa seeds.

Antioxidant Effects
DPPH results of quinoa showed 100.40% compared to vitamin C. As for chia, 90.94% was observed. CUPRAC assay of the seeds showed 934.77 μg/ml and 1109.75 μg/ml BHA concentration for quinoa and chia, respectively.
Weight Management Effect
Body weight of the quinoa group showed no significant change recorded in weeks 1–3, while a significant decrease in weeks 4–6 in comparison to weeks 1 and 2 was observed (Fig. S3). Furthermore, significant decrease in weeks 5 and 6 was reported in comparison to the first 4 weeks. As for chia group, results showed no significant change in body weight of the rats in weeks 1–3, while a significant decrease in body weight of the rats in week 4 in comparison to the first week was observed. Moreover, a significant decrease in body weight in weeks 5 and 6 was seen in comparison to the first 4 weeks. No significant change in body weight of the rats appeared in weeks 1–3 (p > 0.05).
Protection of Liver Enzymes
Rats fed HFD showed more than 2.5-fold increase in ALT and AST levels compared to the negative control group (Group I). Treatment with hydroalcoholic extracts showed significant reduction in liver enzymes ALT and AST by 55.6% and 50.6% for quinoa 62.5% and 58.5% for chia seeds, respectively, as compared to untreated obese rats. No significant difference was observed between the chia, quinoa, and green treated groups (Fig. 1A and B).
Effect of quinoa and chia seeds’ hydroalcoholic extracts on serum: A ALT levels, B AST levels, C cholesterol levels, D triacylglyceride levels, E HDL levels, F LDL levels, G fasting blood glucose levels, H insulin levels. Data are expressed as mean ± SD of three independent experiments (n = 6). Significant differences were identified as follows: asignificantly different from the control group (normal food diet); bsignificantly different from the high-fat diet group; csignificantly different from the reference group at p < 0.05
Lipid Profile Improvement
As depicted in Fig. 1C–F, rats on HFD (Group II) showed significant 91%, onefold and threefold increases in total cholesterol (TC), triacylglycerides (TG), and low-density lipoprotein (LDL) levels, while high-density lipoprotein (HDL) levels were reduced by 54.7% as compared to rats on normal chow diet (Group I), respectively. Treatment with the seeds’ hydroalcoholic extracts improved the lipid profile of rats on HFD compared to the non-treated group, manifested by reduction in TC, TG, and LDL and elevation in HDL levels by 32.5, 43.25, 46, and 64.38% for quinoa and by 33, 30.25, 48.75, and 74.25% for chia extract. Compared to the reference group that received green tea as treatment (Group III), quinoa group showed reduction in TG levels by 25.5%. No significant difference between quinoa (Group IV) and chia extracts (Group V) was detected.
Glycemic Profile Improvement
Rats on HFD showed significant increase in fasting blood glucose (FBG) and serum insulin levels by more than 2.5- fold and 1.3-fold, as compared to the negative control group (Group I). Treatment with quinoa showed ample reduction in FBG and insulin levels by 43.8%. As for chia, reductions of 52.3 and 56.9% have been observed as compared to untreated obese rats, respectively. No significant change in glycemic profiles of green tea (Group III), quinoa (Group IV), and chia (Group V) treated groups was noticed (Fig. 1G and H).
Anti-inflammatory Effect
As illustrated in Fig. 2A, leukotriene LT-B4 levels showed a significant increase in HFD group (Group II) in comparison to rats on normal diet by 225.81%. LT-B4 levels in quinoa and chia groups showed significant decrease in comparison to HFD group by 49.4% and 45.21%, respectively. No significant change between the reference group (HFD + green tea) and the treatment groups was observed. Moreover, both seeds showed the same extent of anti-inflammatory effect.
Effect of quinoa and chia seeds’ hydroalcoholic extracts on serum: A LT–B4 levels, B leptin levels, C adiponectin levels, D MDA levels, E GSH levels. Data are expressed as mean ± SD of three independent experiments (n = 6). Significant differences were identified as follows: asignificantly different from the control (NFD) group; bsignificantly different from the (HFD) group; csignificantly different from the reference (HFD + green tea) group at p < 0.05
Leptin and Adiponectin Modulation
According to Fig. 2B and C, leptin levels were elevated, and adiponectin levels were reduced in HFD group (Group II) obese rats compared to rats on normal diet by 3.6-fold and 74%, respectively. Treatment with the seed extracts reduced leptin levels by 67.2% (quinoa) and 54.26% (chia). On the other hand, adiponectin levels were increased by 1.3-fold and 1.9-fold for quinoa and chia extracts, respectively. No significant change between reference group (HFD + green tea) and treated groups was observed. Noteworthy, quinoa restored leptin levels to normal values of NFD group (Group I). Moreover, both seeds showed the same modulatory effects on leptin and adiponectin levels.
Oxidative Stress Markers
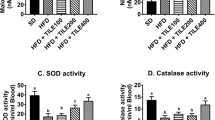
Rats fed with HFD (Group II) showed a significant raise in malondialdehyde (MDA) levels by threefold and reduced glutathione (GSH) levels by 61%, compared to rats on normal diet (Group I). Treatment with the seeds’ extracts reduced MDA levels by 44.5% and 67.3% for the quinoa group and by 37.24% and 62.8% for the chia group, as compared to obese rats treated with green tea (Group III) and the non-treated one (Group II), respectively. The antioxidant marker GSH has been enhanced by onefold following treatment of obese rats with both extracts as compared to the non-treated group. Moreover, GSH levels demonstrated no significant difference between the three herbal groups. Furthermore, no significant difference has been reported between chia and quinoa groups, as compared to the GSH levels in the negative control group (Group I), as shown in Fig. 2D and E.
PPAR-ɣ and AMPK-1α Levels
As illustrated in Fig. 3A and B, PPAR-ɣ and AMPK-1α were reduced in obese rats compared to rats on normal diet by 85.37 and 79%, respectively. Treatment with the seeds reversed this reduction by fourfold for quinoa’s and chia’s PPAR-ɣ levels and by 1.9-fold for quinoa’s and twofold for chia’s AMPK-1α levels. Compared to the reference group (HFD + green tea), quinoa and chia extracts increased the PPAR-ɣ levels by 26.24 and 31.27%, respectively. AMPK-1α levels showed no significant change between the reference group (HFD + green tea) and the treatment groups. Moreover, both seeds showed the same modulatory effects on PPAR-ɣ and AMPK-1α protein levels.
Effect of quinoa and chia seeds’ hydroalcoholic extracts on serum: A PPAR-ɣ, B AMPK-1 levels, C FFA levels, D SERBP levels. Data are expressed as mean ± SD of three independent experiments (n = 6). Significant differences were identified as follows: asignificantly different from the control (NFD) group, bsignificantly different from the (HFD) group, csignificantly different from the reference (HFD + green tea) group, dsignificantly different from the quinoa group at p < 0.05
FFA and SREBP Levels
As depicted in Fig. 3C and D, gene expressions of FFA and SREBP-1c were elevated in obese rats compared to rats on normal diet by sevenfold and 4.7-fold, respectively. Treatment with the seeds curbed the elevation by 65% for quinoa’s and 58% for chia’s FFA levels and by 58.6% for quinoa’s and 44.4% for chia’s SREBP-1 expression levels. It is worth mentioning that the quinoa group showed a greater extent of reduction in FFA expression levels, as compared to the reference group (HFD + green tea) by 29%. SREBP-1 expression levels showed no significant change between the reference group (HFD + green tea) and the treatment groups. Moreover, both seeds showed the same modulatory effects on FFA and SREBP-1 expression levels.
Histopathological Alteration
Liver of rats on HFD revealed histopathological alterations where fibroblastic cell proliferation with inflammatory cells infiltration surrounding the bile duct associated with congestion in the portal vein was demonstrated. Treatment with quinoa showed normal liver architecture similar to the control group with no histopathological alterations. Notably, the protective effect of quinoa on a high-fat diet seemed to exceed the reference and the chia groups, where rats on HFD treated with green tea showed dilatation in the central vein and sinusoids, while the parenchyma showed ballooning degeneration in the hepatocytes with congestion in the sinusoids in between. The chia-treated group showed mild congestion in the central vein, as shown in Fig. 4A–E.
Obesity is considered a global pandemic ascribed to a combination of genetic susceptibility, increased availability of fast foods, and decreased requirement for physical activity in urban life. It should no longer be recognized as simply as a cosmetic challenge for certain individuals, but an epidemic that threatens global health. To date only few approved common therapies are available due to reported limitations, possible tolerance, and unfavorable side effects as diarrhea and abdominal cramps. Thus, an effective safe natural alternative is a pressing need nowadays (Payab et al. 2020; Aron-Wisnewsky et al. 2021). The present study addressed the potential anti-obesity role of commonly prescribed seeds by practitioner for effective diet and better lifestyle: quinoa and chia seeds. This investigation was done via evaluating the PPAR-γ/AMPK-1α signalling pathway and the key-players parameters affecting adipogenesis and lipogenesis in high-fat diet–induced rat model. In correlation with the metabolic complications associated with obesity. Where inflammation and oxidative stress are the main activators in this pathway affecting lipid and glycemic profiles along with liver malfunction. Endowed with their antioxidant, anti-inflammatory, and insulin-sensitizing activities, results of the current work corroborate evidence-based reports to the use of quinoa and chia seeds in weight management. Furthermore, green tea has been chosen as a reference drug as it is reported to be one of the most efficient anti-obesity nutraceuticals. Green tea was proved to have an antioxidant effect through increasing the endogenous antioxidants and decreasing the oxidative stress markers (Zhu et al. 2019). Also, it was found to have a potent anti-inflammatory activity via decreasing the circulating inflammatory cytokines and enhancing the adipokine hormones (Bagheri et al. 2020). Moreover, it was previously reported that green tea enhanced lipid and glycemic profile in test subjects (Asbaghi et al. 2020). Green tea was also reported to exert its anti-obesity activity through the upregulation of PPAR-γ/AMPK-1α signalling pathway (Jiang et al. 2020).
Noteworthy, it has been reported that some obesity-related complications such as inflammation along with oxidative stress, hyperlipidemia, and insulin insensitivity are unequivocal risk factors of metabolic syndrome (Eckel et al. 2005), with higher chances for cardiovascular, hyperlipidemia, type 2 diabetes mellitus, and psychiatric comorbidities (Aballay et al. 2013). Such findings are consistent with results of the present investigation, where rats on HFD showed distorted lipid, glycemic, and adipokine profiles, along with inflammation and oxidative stress. On the other hand, animals treated with both seeds’ extracts effectively reduced body weight compared to the non-treated group. Both extracts amended the lipid, glycemic, oxidative stress, and adipokine profiles of obese rats. They mitigated elevated serum TG and TC content, decreased LDL, and increased HDL levels. Moreover, the extracts were able to enhance GSH and reduce MDA levels; all treatment groups showed a decrease level of insulin and glucose compared to the HFD group. Therefore, our results are consistent with previous research, which stated that the test seeds could modulate lipid profile (Munir et al. 2021; Yao et al. 2021; Oliva et al. 2021).
Notably, obesity results in an altered adipokine profile, with upregulated expression of pro-inflammatory cytokines and leptin, and reduced levels of anti-inflammatory adipokines, such as adiponectin (Hotamisligil 2017). Our outcomes confirmed the ability of the extracts to decrease the level of LT-B4 and leptin, with concurrent increase in adiponectin in test subjects. PPAR-γ, a ligand-dependent transcription factor highly expressed in adipocytes, plays an important role in adipogenesis, lipid storage (Antonopoulos et al. 2016), and insulin sensitivity. Noteworthy, oxidative stress and inflammation are directly linked to PPAR-γ pathway where inflammatory cytokines resulting from increased reactive oxygen species downregulate the expression of PPAR-γ (Algandaby 2020). Our results demonstrated that the seed extracts displayed antioxidant potential by reducing MDA and enhancing GSH, and hence ameliorating obesity by modulating PPAR-γ transcriptional effect and activating the phosphorylation of AMPK-1α. This process is essential for suppression of proteolytic processing, fatty acid oxidation, and transcriptional activity of SREBP-1c which inhibits adipogenesis via promoting lipolysis in adipose tissue and stimulating fatty acid oxidation in the liver (Ho et al. 2019). In addition to its regulation of insulin function, they also regulate the expression of genes controlling free fatty acids, cholesterol, and synthesis of lipids (Damiano et al. 2019). The results here show a clear upregulation of PPAR-γ signalling pathway, which in turn lead to reduction in FFA’s and SREBP-1c levels.
These findings could be further elaborated by the presence of the rich phenolic profile of both seeds. Quinoa seeds are rich in quercetin malonyl-hexoside (5.2%) and kaempferol dirhamnoside (9.21%) which were reported to display antioxidant and anti-inflammatory effects (Cheng et al. 2014; Dabeek and Marra 2019; Kadhum and Thuwaini 2021). On the second hand, these phenolics inhibit lipogenesis and promote lipolysis which in turn suppress lipid accumulation through modulating PPAR-γ/AMPK-α signalling pathway (Khlifi et al. 2020). Furthermore, the presence of dihydroxybenzoic acid-pentoside (7.88%) is reported to have synergetic anti-inflammatory and antioxidant effects (Quaresma et al. 2020), as well as modulating glucose and lipid metabolism (Xochitl et al. 2021). The anti-obesity activity could also be related to the presence of hydroxycinnamic acid (4.95%) and its derivatives including caffeic and ferulic acid hexoside (2.01 and 7.9%, respectively) which were reported to improve lipid metabolism, modulate adipokine hormones (Agregán et al. 2021), and exert antioxidant effect through inhibiting lipid peroxidation and radical scavenging activity (Yuan et al. 2022). Moreover, decreased lipid accumulation and improvement of serum lipid profile could be accredited to the presence of apigenin pentosyl (7.2%) which was reported to inhibit the accumulation of lipids (Bai et al. 2017). Hydroalcoholic extract of quinoa seed was found to be enriched with rosmarinic acid (14.95%) and its derivatives, which were reported to possess lipid lowering effect by decreasing serum total cholesterol and triacylglycerides besides modulating lipid profile. Additionally, it was found to inhibit α-glucosidase which in turn inhibits lipid accumulation (Zhou et al. 2021). Also, rosmarinic acid upregulates PPAR-γ levels, and henceforth decreasing adipogenesis in coordination with decreasing inflammation by modulating adipokine hormones (Vasileva et al. 2021).
Chia seeds showed a high content of salvianolic acid C (13.8%), as the major dereplicated antioxidant, which showed a potent α-glucosidase inhibitory activity with IC50 of 3.03 ± 0.27 μM (Tang et al. 2019). Also, this polyphenol inhibited LPS-induced inflammatory response and NF-κB activation through the activation of AMPK/Nrf2 signaling both in vivo and in vitro (Song et al. 2018). In addition, a high content of malic acid was found (7.49%), which is reported to be a potent lipase inhibitor, where it decreases the fat absorption in the intestine which in turn improves the lipid profile (Porras-Mija et al. 2020). Moreover, the presence of syringic acid and caffeic acid and their glycosides (3.89, 2.01%, respectively) contribute to slim-aid effect of chia due to inhibitory effect on α-amylase and lipase enzymes, which modulate adipogenesis and lipogenesis process in the body (Hegazi et al. 2020). Owing to the presence of danshensu glucuronide (13.83%) which suppresses ROS formation, decreases lipid peroxidation, and promotes anti-inflammatory effects (Nicolì et al. 2019) and act as an PPAR-γ agonist, hence upregulates PPAR-γ/AMPK-α signalling pathway leading to modulation of adipogenesis along with decreasing insulin resistance and improving glycemic profile (Xu et al. 2017).
Morbid obesity enhances accumulation of fats on liver, which eventually leads to liver fibrosis and histopathological complications (Xiong et al. 2017). Our hepatic histopathological findings of obese rats showed drastic damage in the portal vein and hepatocytes rupture with high severity along with elevated liver enzymes in comparison to seed extracts–treated groups. This goes in harmony with previous studies which showed the hepatoprotective effect of quinoa (Saxena et al. 2017) and chia seeds (Apoorva et al. 2020). The presence of salvianolic acid C (13.79%) and ferulic acid hexoside (7.9%) in the quinoa-treated group could be a reason behind its hepatoprotective effects, in line with Wu et al. (2019) and Peng et al. (2021). As for chia seeds, the presence of caffeic acid derivative (22.59%), rosmarinic acid (14.95%), and malic acid (7.49%) could be the reason behind the observed hepatoprotective profile as previously described (Kaur et al. 2019; Hiraishi et al. 2022).
Conclusion
The present study evidenced that supplementation with quinoa and chia reduced body weight gain, contributed to control blood glycemic profile, and decreased the atherogenic index of plasma. Both seeds’ supplementation reduced adipogenesis through the downregulation of adipogenesis transcription factors and lipogenesis mediators, in addition to their upregulatory effect of oxidative-encoding genes and downregulatory effect of lipogenic-related genes. Results hypothesized the potential applicability of the test seeds components in the prevention of obesity and its related disorder, introducing to medical community and food industry an economic and alternative safe approach to halt metabolic syndrome comorbidities.
Data Availability
The data to support the findings of this study are included within the article or in the supplementary data.
References
Aballay LR, Eynard AR, Díaz MP, Navarro A, Muñoz SE (2013) Overweight and obesity: a review of their relationship to metabolic syndrome, cardiovascular disease, and cancer in South America. Nutr Rev 71:168–179. https://doi.org/10.1111/j.1753-4887.2012.00533.x
Agregán R, Munekata PE, Feng X, Astray G, Gullón B, Lorenzo JM (2021) Recent advances in the extraction of polyphenols from eggplant and their application in foods. LWT 146:111381. https://doi.org/10.1016/j.lwt.2021.111381
Ahmed W, Ziouzenkova O, Brown J, Devchand P, Francis S, Kadakia M, Kanda T, Orasanu G, Sharlach M, Zandbergen F (2007) PPARs and their metabolic modulation: new mechanisms for transcriptional regulation? J Intern Med 262:184–198. https://doi.org/10.1111/j.1365-2796.2007.01825.x
Algandaby MM (2020) Crocin prevents metabolic syndrome in rats via enhancing PPAR-gamma and AMPK. Saudi J Biol Sci 27:1310–7316. https://doi.org/10.1016/j.sjbs.2020.01.004
Antonopoulos AS, Margaritis M, Verheule S, Recalde A, Sanna F, Herdman L, Psarros C, Nasrallah H, Coutinho P, Akoumianakis I, Brewer AC (2016) Mutual regulation of epicardial adipose tissue and myocardial redox state by PPAR-γ/adiponectin signalling. Circ Res 118:842–855. https://doi.org/10.1161/CIRCRESAHA.115.307856
Apak R, Güçlü K, Özyürek M, Esin Karademir S, Erçağ E (2006) The cupric ion reducing antioxidant capacity and polyphenolic content of some herbal teas. Int J Food Sci Nutr 57:292–304. https://doi.org/10.1080/09637480600798132
Apoorva N, Rao RR, Shenoy PJ, Sindhu H, Teerthanath S (2020) Salvia hispanica (chia) seeds afford hepatoprotection against isoniazid and rifampicin induced toxicity in a murine model. Res J Pharm Technol 13:4805–4810. https://doi.org/10.5958/0974-360X.2020.00845.8
Apovian CM, Aronne LJ, Bessesen DH, McDonnell ME, Murad MH, Pagotto U, Ryan DH, Still CD (2015) Pharmacological management of obesity: an endocrine society clinical practice guideline. J Clin Endocr Metab 100:342–362. https://doi.org/10.1210/jc.2014-3415
Aron-Wisnewsky J, Warmbrunn MV, Nieuwdorp M, Clément (2021) Metabolism and metabolic disorders and the microbiome: the intestinal microbiota associated with obesity, lipid metabolism, and metabolic health—pathophysiology and therapeutic Strategies Gastroenterol 160:573–599. https://doi.org/10.1053/j.gastro.2020.10.057
Aryal S, Baniya MK, Danekhu K, Kunwar P, Gurung R, Koirala N (2019) Total phenolic content, flavonoid content and antioxidant potential of wild vegetables from western Nepal. Plants 8:96. https://doi.org/10.3390/plants8040096
Asbaghi O, Fouladvand F, Moradi S, Ashtary-Larky D, Choghakhori R, Abbasnezhad A (2020) Effect of green tea extract on lipid profile in patients with type 2 diabetes mellitus: a systematic review and meta-analysis. Diabetes Metabol Syndr Clin Res Rev 14:293–301. https://doi.org/10.1016/j.dsx.2020.03.018
Ascaso JF, Romero P, Real JT, Lorente RI, Martı́nez-Valls J, Carmena R, (2003) Abdominal obesity, insulin resistance, and metabolic syndrome in a Southern European population. Eur J Intern Med 14:101–106. https://doi.org/10.1016/S0953-6205(03)00022-0
Bagheri R, Rashidlamir A, Ashtary-Larky D, Wong A, Alipour M, Motevalli MS, Chebbi A, Laher I, Zouhal H (2020) Does green tea extract enhance the anti-inflammatory effects of exercise on fat loss? Br J Clin Pharmacol 86:753–762. https://doi.org/10.1111/bcp.14176
Bai WX, Wang C, Wang YJ, Zheng WJ, Wang W, Wan XC, Bao GH (2017) Novel acylated flavonol tetraglycoside with inhibitory effect on lipid accumulation in 3T3-L1 cells from Lu’an GuaPian tea and quantification of flavonoid glycosides in six major processing types of tea. J Agric Food Chem 65:2999–3005. https://doi.org/10.1021/acs.jafc.7b00239
Bancroft JD, Gamble M (1996) Theory and practice of histological techniques. New York: Churchill Livingstone 4
Barish GD, Narkar VA, Evans RM (2006) PPARδ: a dagger in the heart of the metabolic syndrome. J Clin Investig 116:590–597. https://doi.org/10.1172/JCI27955
Chanda D, Lee CH, Kim YH, Noh JR, Kim DK, Park JH, Hwang JH, Lee MR, Jeong KH, Lee IK, Kweon GR (2009) Fenofibrate differentially regulates plasminogen activator inhibitor-1 gene expression via adenosine monophosphate–activated protein kinase–dependent induction of orphan nuclear receptor small heterodimer partner. J Hepatol 50:880–892. https://doi.org/10.1074/jbc.M110.134890
Cheng DM, Pogrebnyak N, Kuhn P, Krueger CG, Johnson WD, Raskin I (2014) Development and phytochemical characterization of high polyphenol red lettuce with anti-diabetic properties. PLoS ONE 9:e91571. https://doi.org/10.1371/journal.pone.0091571
Dabeek WM, Marra MV (2019) Dietary quercetin and kaempferol: bioavailability and potential cardiovascular-related bioactivity in humans. Nutrients 11:2288. https://doi.org/10.3390/nu11102288
Damiano F, Giannotti L, Gnoni GV, Siculella L, Gnoni A (2019) Quercetin inhibition of SREBPs and ChREBP expression results in reduced cholesterol and fatty acid synthesis in C6 glioma cells. Int J Biochem Cell Biol 117:105618. https://doi.org/10.1016/j.biocel.2019.105618
De Falco B, Amato M, Lanzotti V (2017) Chia seeds oroducts: an overview. Phytochem Rev 16:745–760. https://doi.org/10.1007/s11101-017-9511-7
Eckel RH, Grundy SM, Zimmet PZ (2005) The metabolic syndrome. Lancet 365:1415–1428. https://doi.org/10.1016/S0140-6736(05)66378-7
Elshamy S, Abdel Motaal A, Abdel-Halim M, Medhat D, Handoussa H (2021). Potential neuroprotective activity of Mentha longifolia L. in aluminum chloride-induced rat model of Alzheimer’s disease. J Food Biochem 45:1770. https://doi.org/10.1111/jfbc.13644
Emanuela F, Grazia M, Marco DR, Maria Paola L, Giorgio F, Marco B (2012) Inflammation as a link between obesity and metabolic syndrome. J Nutr Metab 2012:476380. https://doi.org/10.1155/2012/476380
Fassina P, Adami FS, Zani VT, Machado IC, Garavaglia J, Grave MT, Ramos R, Dal Bosco SM (2015) The effect of Garcinia cambogia as coadjuvant in the weight loss process. Nutr Hosp 32:2400–2408. https://doi.org/10.3305/nh.2015.32.6.9587
Ghahremanloo A, Hajipour R, Hemmati M, Moossavi M, Mohaqiq Z (2018) The beneficial effects of pumpkin extract on atherogenic lipid, insulin resistance and oxidative stress status in high-fat diet-induced obese rats. J Complement Integr Med 15:20170051. https://doi.org/10.1515/jcim-2017-0051
Hegazi NM, Radwan RA, Bakry SM, Saad HH (2020) Molecular networking aided metabolomic profiling of beet leaves using three extraction solvents and in relation to its anti-obesity effects. J Adv Res 24:545–555. https://doi.org/10.1016/j.jare.2020.06.001
Hill JO, Peters JC (1998) Environmental contributions to the obesity epidemic. Science 280:1371–1374. https://doi.org/10.1126/science.280.5368.1371
Hiraishi K, Jimma F, Soma H, Kagawa T, Yamaoka I (2022) Investigating a novel hepatoprotective substance from ume extract (heated Japanese apricot juice concentrate). Part 1: finding an active substance using a liver injury rat model. NFS J 26:22–32. https://doi.org/10.1016/j.nfs.2021.05.003
Ho C, Gao Y, Zheng D, Liu Y, Shan S, Fang B, Zhao Y, Song D, Zhang Y, Li Q (2019) Alisol A attenuates high-fat-diet-induced obesity and metabolic disorders via the AMPK/ACC/SREBP-1c pathway. J Cell Mol Med 23:5108–5118. https://doi.org/10.1111/jcmm.14380
Hotamisligil GS (2017) Foundations of immunometabolism and implications for metabolic health and disease. Immunity 47:406–420. https://doi.org/10.1016/j.immuni.2017.08.009
Jiang H, Horiuchi Y, Hironao KY, Kitakaze T, Yamashita Y, Ashida H (2020) Prevention effect of quercetin and its glycosides on obesity and hyperglycemia through activating AMPKα in high-fat diet-fed ICR mice. J Clin Biochem Nutr 67:75–83. https://doi.org/10.3164/jcbn.20-47
Kadhum HS, Thuwaini MM (2021) Anti-obesity and hypolipidemic effects of Morus alba - a review. GSC Biol Pharm Sci 17:186–192. https://doi.org/10.30574/gscbps.2021.17.1.0316
Kaur KK, Allahbadia GN, Singh M (2019) Rosmarinic acid - a new hope for liver diseases like cirrhosis, hepatocellular carcinoma-needs translation to humans. EC Endocrinol Met Res 4:289–301
Kazemipoor M, Radzi CW, Cordell GA, Yaze I (2012) Potential of traditional medicinal plants for treating obesity: a review. ArXiv Preprint ArXiv:1208.1923. https://doi.org/10.48550/arXiv.1208.1923
Khlifi R, Dhaouefi Z, Toumia IB, Lahmar A, Sioud F, Bouhajeb R, Bellalah A, Chekir-Ghedira L (2020) Erica multiflora extract rich in quercetin-3-O-glucoside and kaempferol-3-O-glucoside alleviates high fat and fructose diet-induced fatty liver disease by modulating metabolic and inflammatory pathways in Wistar rats. J Nutr Biochem 86:108490. https://doi.org/10.1016/j.jnutbio.2020.108490
Kulczyński B, Kobus-Cisowska J, Taczanowski M, Kmiecik D, Gramza-Michałowska A (2019) The chemical composition and nutritional value of chia seeds—current state of knowledge. Nutr J 11:1242. https://doi.org/10.3390/nu11061242
Lage R, Diéguez C, Vidal-Puig A, López M (2008) AMPK: a metabolic gauge regulating whole-body energy homeostasis. Trends Mol Med 14:539–549. https://doi.org/10.1016/j.molmed.2008.09.007
Liu M, Yun P, Hu Y, Yang J, Khadka RB, Peng X (2020a) Effects of grape seed proanthocyanidin extract on obesity. Obes Facts 2:279–291. https://doi.org/10.1159/000502235
Liu M, Zhu K, Yao Y, Chen Y, Guo H, Ren G, Yang X, Li J (2020b) Antioxidant, anti-inflammatory, and antitumor activities of phenolic compounds from white, red, and black Chenopodium quinoa seed. Cereal Chem 97:703–713. https://doi.org/10.1002/cche.10286
Magkos F, Fraterrigo G, Yoshino J, Luecking C, Kirbach K, Kelly SC, de Las FL, He S, Okunade AL, Patterson BW, Klein S (2016) Effects of moderate and subsequent progressive weight loss on metabolic function and adipose tissue biology in humans with obesity. Cell Metab 23:591–601. https://doi.org/10.1016/j.cmet.2016.02.005
Munir S, Khurshid S, Iqbal QJ, Iqbal N, Masood Z (2021) Effect of basil seed and chia seed extracts on blood lipid profile. Pak J Med Health Sci 15:2117–2120. https://doi.org/10.53350/pjmhs211582117
Murase T, Misawa K, Minegishi Y, Aoki M, Ominami H, Suzuki Y, Shibuya Y, Hase T (2011) Coffee polyphenols suppress diet-induced body fat accumulation by downregulating srebp-1c and related molecules in C57BL/6J mice. Am J Physiol Endocrinol 300:E122–E133. https://doi.org/10.1152/ajpendo.00441.2010
Nicolì F, Vergine M, Negro C, Luvisi A, Nutricati E, Aprile A, Rampino P, Sabella E, De Bellis L, Miceli A (2019) Salvia clandestina L.: unexploited source of Danshensu. Nat Prod Res 33:439–442. https://doi.org/10.1080/14786419.2018.1452015
Noratto GD, Murphy K, Chew BP (2019) Quinoa intake reduces plasma and liver cholesterol, lessens obesity-associated inflammation, and helps to prevent hepatic steatosis in obese Db/Db mouse. Food Chem 287:107–114. https://doi.org/10.1016/j.foodchem.2019.02.061
Oliva ME, del Rosario Ferreira M, Joubert MB, D'Alessandro ME (2021) Salvia hispanica L. (chia) seed promotes body fat depletion and modulates adipocyte lipid handling in sucrose-rich diet-fed rats. Int Food Res J 139:109842. https://doi.org/10.1016/j.foodres.2020.109842
Payab M, Hasani-Ranjbar S, Shahbal N, Qorbani M, Aletaha A, Haghi-Aminjan H, Soltani A, Khatami F, Nikfar S, Hassani S, Abdollahi M (2020) Effect of the herbal medicines in obesity and metabolic syndrome: a systematic review and meta-analysis of clinical trials. Phytother Res 34:526–545. https://doi.org/10.1002/ptr.6547
Peng J, Xie J, Shi S, Luo L, Li K, Xiong P, Cai W (2021) Diagnostic fragment-ion-based for rapid identification of chlorogenic acids derivatives in Inula cappa Using UHPLC-Q-Exactive orbitrap mass spectrometry. J Anal Methods Chem 2021:6393246. https://doi.org/10.1155/2021/6393246
Porras-Mija I, Chirinos R, Garcia-Rios D, Aguilar-Galvez A, Huaman-Alvino C, Pedreschi R, Campos D (2020) Physico-chemical characterization, metabolomic profile and in vitro antioxidant, antihypertensive, antiobesity and antidiabetic properties of Andean elderberry (Sambucus nigra subsp. peruviana). J Berry Res 10:193–208. https://doi.org/10.3233/JBR-190439
Quaresma DM, Justino AB, Sousa RM, Munoz RA, de Aquino FJ, Martins MM, Goulart LR, Pivatto M, Espindola FS, de Oliveira A (2020) Antioxidant compounds from Banisteriopsis argyrophylla leaves as α-amylase, α-glucosidase, lipase, and glycation inhibitors. Bioorg Chem 105:104335. https://doi.org/10.1016/j.bioorg.2020.104335
Rasheed H, Shehzad M, Rabail R, Kowalczewski PŁ, Kidoń M, Jeżowski P, Ranjha MM, Rakha A, Din A, Aadil RM (2022) Delving into the nutraceutical benefits of purple carrot against metabolic syndrome and cancer: a review. J Appl Sci 12:3170. https://doi.org/10.3390/app12063170
Saxena SN, Shahani LA, Bhatnagar PR (2017) Hepatoprotective effect of Chenopodium quinoa seed against CCL4-induced liver toxicity in Swiss albino male mice. Asian J Pharm Clin Res 10:273–76. https://doi.org/10.22159/ajpcr.2017.v10i11.20918
Scapin G, Schmidt MM, Prestes RC, Rosa CS (2016) Phenolics compounds, flavonoids and antioxidant activity of chia seed extracts (Salvia hispanica) obtained by different extraction conditions. Int Food Res J 23:2341–2346
Shalaby RH, Rashed LA, Ismaail AE, Madkour NK, Elwakeel SH (2014) Hematopoietic stem cells derived from human umbilical cord ameliorate cisplatin-induced acute renal failure in rats. Am J Stem Cells 383–96.
Song J, Zhang W, Wang J, Yang H, Zhao X, Zhou Q, Wang H, Li L, Du G (2018) Activation of Nrf2 signaling by salvianolic acid C attenuates NF-κB mediated inflammatory response both in vivo and in vitro. Int Immunopharmacol 63:299–310. https://doi.org/10.1016/j.intimp.2018.08.004
Tang H, Ma F, Zhao D, Xue Z (2019) Exploring the effect of salvianolic acid C on α-glucosidase: inhibition kinetics, interaction mechanism and molecular modelling methods. Process Biochem 78:178–188. https://doi.org/10.1016/j.procbio.2019.01.011
Vanhanen M, Koivisto K, Moilanen L, Helkala EL, Hänninen T, Soininen H, Kervinen K, Kesäniemi YA, Laakso M, Kuusisto J (2006) Association of metabolic syndrome with Alzheimer disease: a population-based study. J Neurol 67:843–847. https://doi.org/10.1212/01.wnl.0000234037.91185.99
Vasileva LV, Savova MS, Tews D, Wabitsch M, Georgiev MI (2021) Rosmarinic acid attenuates obesity and obesity-related inflammation in human adipocytes. Food Chem Toxcol 149:112002. https://doi.org/10.1016/j.fct.2021.112002
Wu CT, Deng JS, Huang WC, Shieh PC, Chung MI, Huang GJ (2019) Salvianolic acid C against acetaminophen-induced acute liver injury by attenuating inflammation, oxidative stress, and apoptosis through inhibition of the Keap1/Nrf2/HO-1 signaling. Oxid Med Cell Longev 2019:9056845. https://doi.org/10.1155/2019/9056845
Xiong DD, Zhang M, Li N, Gai JF, Mao L, Li M (2017) Mediation of inflammation, obesity and fatty liver disease by advanced glycation endoproducts. Eur Rev Med Pharmacol Sci 21:5172–8178. https://doi.org/10.26355/eurrev_201711_13835
Xochitl AF, Rosalía RC, Minerva RG, Mendoza-Sánchez M, Mora O, Pérez-Ramírez IF (2021) Polyphenols and avenanthramides extracted from oat (Avena sativa L.) grains and sprouts modulate genes involved in glucose and lipid metabolisms in 3T3 L1 adipocytes. J Food Biochem 45:e13738. https://doi.org/10.1111/jfbc.13738
Xu P, Hong F, Wang J, Wang J, Zhao X, Wang S, Xue T, Xu J, Zheng X, Zhai Y (2017) DBZ is a putative PPARγ agonist that prevents high fat diet-induced obesity, insulin resistance and gut dysbiosis. BBA-Gen Subj 1861:2690–2701. https://doi.org/10.1016/j.bbagen.2017.07.013
Yao D, Zhang B, Zhu J, Zhang Q, Hu Y, Wang S, Wang Y, Cao H, Xiao J (2020) Advances on application of fenugreek seeds as functional foods: pharmacology, clinical application, products, patents and market. Crit Rev Food Sci Nutr 60:2342–2352. https://doi.org/10.1080/10408398.2019.1635567
Yao N, Yan S, Guo Y, Wang H, Li X, Wang L, Hu W, Li B, Cui W (2021) The association between carotenoids and subjects with overweight or abesity: a systematic review and meta-analysis. Food Funct 12:4768–4782. https://doi.org/10.1039/d1fo00004g
Yuan Y, Xiang J, Zheng B, Sun J, Luo D, Li P, Fan J (2022) Diversity of phenolics including hydroxycinnamic acid amide derivatives, phenolic acids contribute to antioxidant properties of proso millet. LWT 154:112611. https://doi.org/10.1016/j.lwt.2021.112611
Zhou L, Ding X, Wang J, Bai S, Zeng Q, Su Z, Xuan Y, Zhang K (2021) Tea polyphenols increase the antioxidant status of laying hens fed diets with different levels of ageing corn. Animal Nutr 7:650–660. https://doi.org/10.1016/j.aninu.2020.08.013
Zhu F, Wang J, Takano H, Xu Z, Nishiwaki H, Yonekura L, Yang R, Tamura H (2019) Rosmarinic acid and its ester derivatives for enhancing antibacterial, α-glucosidase inhibitory, and lipid accumulation suppression activities. J Food Biochem 43:e12719. https://doi.org/10.1111/jfbc.12719
Acknowledgements
The authors are grateful to their institutions for the opportunity to develop this publication.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
NHO: investigation, formal analysis, methodology, manuscript writing, and proofreading. AAZE-B: formal analysis, methodology, manuscript writing, and proofreading. HTAH: formal analysis. HH: setting the protocol, practical supervision, formal analysis, methodology, manuscript writing, and proofreading.
Corresponding author
Ethics declarations
Ethical Approval
Experimental procedures were conducted in accordance with the Egyptian National Institutional Guidelines on Animal Experimentation and approved by the Animals Ethics Committee at the GUC (#PB35879) obtained 28.6.2020 in association with the recommendations of the National Institutes of Health (NIH) Guide for Care and Use of Laboratory Animals (Publication No. 85–23, revised 1985).
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Omran, N.H., El-Bahy, A.A.Z., Hosny, H.T.A. et al. Quinoa and Chia Modulate AMPK/PPAR-ɣ Signaling in High-Fat Diet–Induced Obesity Rat Model. Rev. Bras. Farmacogn. 33, 583–594 (2023). https://doi.org/10.1007/s43450-023-00388-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43450-023-00388-5