Abstract
In vitro propagation of yam via organogenesis is constrained with low multiplication rate. Somatic embryogenesis (SE) has shown rapid multiplication potentials in yam. However, it has not been adopted by practical seed system scenarios due to genotype specificity. Reports have shown that SE is regulated endogenously by phytochemicals, but this is yet to be elucidated for yam. This study identified, quantified endogenous, and evaluated effects of exogenous application of selected identified phytochemicals in yam SE. Callus was induced from in vitro axillary bud explants of three Dioscorea rotundata genotypes in Murashige and Skoog (MS) medium containing 9.1 µM 2,4-dichlorophenoxyacetic acid and 5.4 µM naphthaleneacetic acid. Plantlets were regenerated using MS medium containing 4.4 µM benzylaminopurine and 34.0 µM uniconazole-P. Endogenous phytochemicals associated with axillary bud, calluses, and plantlets were identified and quantified using GC/MS. Effect of selected identified phytochemicals on the genotypes was investigated in a 5 × 6 factorial in completely randomized design (r = 3). Data taken on plantlet regeneration was analyzed using ANOVA at α0.05. A total of 27, 22, and 35 phytochemicals were identified in Kpamyo, Ekiti2a, and Asiedu, respectively. Hexamethylcyclotrisiloxane (36.4%, Kpamyo), Tris-tert-butyldimethylsilyloxy-arsane (59.3%, Ekiti2a), and 4-methyl-2-trimethylsililoxy-acetophenone (52.7%, Asiedu) were highest in callus. N-Methyl-1-adamantaneacetamide (31.8%, Kpamyo) and Tris-tert-butyldimethylsilyloxy-arsane (52.7%, Ekiti2a, Asiedu) were highest in plantlets while Tris-tert-butyldimethylsilyloxy-arsane (41.2%, Kpamyo), hexamethylcyclotrisiloxane (55.8%, Ekiti2a), and erythro-9,10-dibromopentacosane (38.9%, Asiedu) were highest in axillary bud. Plantlet regeneration differed significantly among phytochemicals and ranged from 0.7 ± 0.3 (control) to 4.5 ± 0.5 (40.5 µM phenylacetic acid). Also, genotype × phytochemical interactions on number of plantlets regenerated were significant, and mean values ranged from 0.0 ± 0.0 (TDa2014, 4.8 µM decamethyltetrasiloxane) to 7.0 ± 1.7 (TDa2014, 40.5 µM phenylacetic acid). The application of 40.5 µM phenylacetic acid enhanced plantlet regeneration in Kpamyo and TDa2014 by 5.39% and 343.04%, respectively.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Yam is a multi-species crop that is mostly propagated using the tuber part, which is also the edible part (Adeigbe et al. 2015). Despite the importance of yam in alleviating food insecurity in Africa, the availability of clean planting material remains a major challenge due to its low propagation ratio, coupled with the long periods of tuber dormancy (Maroya et al. 2014). Various technologies have been investigated for increase in yam propagation rates. These include the advanced yam minisett technology (planting cut yam tubers of 25 to 50 g size range), rooted vine cuttings, the temporary immersion bioreactor system of tissue culture, and aeroponics and hydroponics system (Maroya et al. 2014; Balogun et al. 2017, 2021).
Tissue culture technique has the advantage of cleaning infected propagules from pathogens hitherto accumulated during cultivation in the informal seed system (Coyne et al. 2009; Balogun et al. 2014). However, the use of tissue culture technique through organogenesis is still constrained with low multiplication ratio (1:4). Somatic embryogenesis (SE) (the production of embryos from somatic cells and subsequent conversion of the embryos to plantlets) has been successfully used by many researchers to increase this limited multiplication in different crops (Junaid et al. 2013; Ossai et al. 2018). However, rates of SE are affected by genotype, type and concentration of growth regulators in culture medium, photoperiod, type, and stage of explants and endogenous phytochemicals that mediate hormone actions in various plant developmental stages and their morphogenic processes (Kakkar and Sawhney 2002).
Yam is rich in different levels of hormonally active phytochemicals (Polycarp et al. 2012) which could play different roles in their competence to SE as seen in other plants. Plants typically release some of these phytochemicals into the environment in response to alterations in the ambient conditions, whether harmful or beneficial, a condition known as plasticity (Loretta 2014). According to Graf et al. (2007), plant response to tissue culture is controlled largely by the epigenetic changes (methylation or demethylation) exhibited by the plants at different stages of regeneration pathway (organogenesis or somatic embryogenesis).
In somatic embryogenesis pathway, several genes that regulate oxidative stresses in plants (Santos et al. 2018) characterize the somatic embryo induction stage. This is because environmental stressors stimulate the callus induction stage, which leads to the production of reactive oxygen species (ROS) or pro-oxidants. These ROS under normal conditions are controlled by antioxidants, which are endogenic (phytochemicals) in plants (Joseph and Jini 2010). However, in a stress condition, there is an imbalance between the production and elimination of ROS in plants, and the capability to balance the redox varies among and within plant species, a situation that could necessitate the exogenic application of the antioxidants to balance the system experimentally. Proteomics is a veritable tool to understand these phytochemicals and their control mechanisms (Ahmad et al. 2016). Knowledge of these phytochemicals is important in adjusting the media compositions to enhance SE in otherwise incompetent genotypes. This study identified, quantified endogenous, and exogenically evaluated effects of selected identified phytochemicals during stages of SE in white (Kpamyo, Ekiti2a and Asiedu) and water (TDa2014 and Swaswa) yams.
Materials and Methods
Study Location
The tissue culture experiments were carried out at the Cell Biology Unit of the Bioscience Center, International Institute of Tropical Agriculture (IITA), while the identification of expressed phytochemicals during somatic embryogenesis was carried out at the Central Research Laboratory, Tanke Road, Ilorin, Nigeria. The equipment were sourced from the central store, supply chain unit of IITA, Ibadan, Nigeria, while the PGRs and phytochemicals were sourced from Bristol Scientific, a Sigma-Aldrich distributor in Lagos, Nigeria.
Three Dioscorea rotundata Poir (Asiedu, Ekiti2a, and Kpamyo) and two Dioscorea alata L. (Swaswa and TDa2014) genotypes were used for the somatic embryogenesis evaluation. Asiedu, Ekiti2a, and Kpamyo were used for phytochemical profiling, while Asiedu, Ekiti2a, Kpamyo, Swaswa, and TDa2014 were used for evaluating selected expressed phytochemicals during somatic embryogenesis. To induce callus, in vitro axillary bud explants (0.1 × 0.5 cm2) excised from 2-wk-old organogenesis-derived plantlets of Asiedu, Ekiti2a, and Kpamyo were cultured in Murashige and Skoog (MS) medium (Murashige and Skoog 1962; callus induction medium (CIM)) and modified with 9.1 µM 2,4-dichlorophenoxylacetic acid and 5.4 µM naphthaleneacetic acid under laminar flow hood. The cultures were incubated in the dark for four (4) wk at 25 ± 1 °C. The induced calluses were transferred to plant growth regulator (PGR)–free MS medium and incubated at 16-h photoperiod producing 4000 lx of light and 25 ± 1 °C for three wk for the formation of somatic embryos. The somatic embryos were thereafter transferred to MS medium modified with 4.4 µM benzylaminopurine and 34 µM uniconazole-P (plantlet regeneration medium (PRM)) and kept at 16-h photoperiod and 25 ± 1 °C.
Identification of Endogenous Phytochemicals During SE Stages of Three Genotypes of White Yam (D. rotundata Poir) (Fig. 1)
Organogenesis-derived axillary bud 4-wk-old callus and 8-wk-old callus cultures at plantlet regeneration stage of Asiedu, Ekiti2a, and Kpamyo were collected for phytochemical profiling and quantification following the protocol of Saleethong et al. (2016) and Bradford (1976—Bradford protein assay), respectively. The protein samples were lyophilized and derivatized by the addition of 5 µL hexadecane, 73.0 µL dimethylformamide, 20.0 µL N-tert-butyldimethylsilyl-N-methyltriflouroacetamide, and 5.0 µL of triethylalanine (catalyst) at a temperature of 60 °C and left for 30 min. The derivatized samples were injected into a gas chromatograph, heated up to 300 °C where the material was volatilized to enable separation of components that flow through the column by size for phytochemical detection. The data generated were searched against the National Institute of Standards and Technology (NIST) and the National Center for Biotechnology Information (NCBI) databases for phytochemical identification and the respective percentage of the sample covered by each phytochemical detected.
Decamethyltetrasiloxane (DT), hexamethylcyclotrisiloxane (HC), phenylacetic acid (PAA), and glutaric acid (GLA) were quantified prior to investigation of effects of their exogenous application on SE. In endogenous determination in tissue cultures, 1 L of callus induction medium was dispensed into 60 Petri plates of 16.7 mL each. Five (5) axillary bud explants were cultured in 16.7 mL of callus induction medium for callus formation. Hence, weight (g) of phytochemical / liter = detected weight of phytochemical (g) per callus × 5 × 60 (Table 1). Selected DT and HC were found across the three genotypes in the 4-wk-old callus culture, and PAA was detected across the three genotypes in the 8-wk-old callus cultures, while GLA was detected across the genotypes in the organogenesis-derived axillary bud. The phytochemicals were sourced from Sigma-Aldrich and added to the callus induction and plantlet regeneration media for Asiedu, Ekiti2a, Kpamyo, Swaswa, and TDa2014 genotypes.
Callus induction medium (CIM) was prepared and the selected phytochemicals were added to give six (6) treatments (T): T1: CIM, T2: CIM plus 2.1 µM GLA, T3: CIM plus 28.5 µM PAA, T4: CIM plus 95.9 µM HC, T5: CIM plus 9.6 µM DT, and T6: yam multiplication medium (YMM). The six culture medium regimes were autoclaved at 121 °C and 15 Psi for 15 min and dispensed into Petri plates (16.7 mL each) under the laminar flow hood. Five (5) in vitro axillary buds (0.1 to 0.5 cm) of Asiedu, Kpamyo, Ekiti2a, Swaswa, and TDa2014 were excised and inoculated into Petri plates containing 16.7 mL of the different callus induction media and replicated three times per genotype. The cultures were kept in the dark for callus formation and proliferation (Manoharan et al. 2016).
The calluses were transferred to PGR-free YMM and kept in a 16-h photoperiod at 25 ± 1 °C condition for 3 wk. During this period, the cultures were assessed for the formation and maturation of somatic embryos by aseptically viewing the cultures under a light microscope. For plantlet regeneration, the phytochemicals were added to the PRM in the following treatment combinations; M1: PRM, M2: PRM plus 2.1 µM GLA, M3: PRM plus 40.5 µM PAA, M4: PRM plus 20.4 µM HC, M5: PRM plus 4.8 µM DT, and M6: YMM. The six medium regimes were autoclaved at 121 °C and 15 Psi for 15 min and dispensed into Petri plates in 16.7 mL quantities. The embryogenic calluses were transferred into the medium. In each of callus induction and plantlet regeneration studies, a factorial 5 (genotypes) by 6 (medium regimes) in completely randomized design (CRD) with 3 replicates was used. Data were collected on the number of days to callus induction, percentage callus formation, days to plantlet regeneration, number of plantlets regenerated per callus, and number of roots formed. Data were analyzed using correlation (between quantity of identified phytochemicals by number of plantlets regenerated) and ANOVA (SAS 9.0 version), and treatment means were separated using the Duncan multiple range test (DMRT) at p ≤ 0.05.
Results
Competence of Selected Genotypes of White and Water Yam for Somatic Embryogenesis (Figs. 2 and 3)
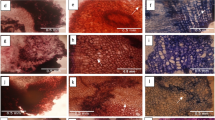
Genotype × phytochemicals interaction on days to callus induction and percentage callus induction in Dioscorea species. CIM, callus induction medium. (A) CIM plus 2.1 µM glutaric acid. (B) CIM. (C) CIM + 28.5 µM phenylacetic acid. (D) Yam multiplication medium. (E) CIM plus 95.9 µM .hexamethylcyclotrisiloxane. (F) CIM + 9.6 µM decamethyltetrasiloxane.
Genotype × phytochemicals interaction on plantlet regeneration in Dioscorea species through somatic embryogenesis. PRM, plantlet regeneration medium. (A) PRM plus 2.1 µM glutaric acid. (B) PRM. (C) PRM plus 40.5 µM phenylacetic acid. (D) Yam multiplication medium. (E) PRM plus 20.4 µM hexamethylcyclotrisiloxane. (F) CIM plus 4.8 µM decamethyltetrasiloxane.
Asiedu took the longest number of day (23.00 ± 2.43) to induce callus; however, it was not significantly different from the rest of the genotypes (Table 2). Also, Asiedu (100 ± 0.13%) had the highest percentage of callus formation which was not significantly different from Kpamyo (90 ± 0.13%) and Ekiti2a (80.00 ± 0.13%) but was significantly higher than TDa2014 (50.00 ± 0.13%) and Swaswa (35.00 ± 0.13%). The number of days to regenerate plantlet in Swaswa (76.00 ± 2.66) was not significantly different from that in Kpamyo (73.25 ± 2.66), Asiedu (72.00 ± 2.66), and TDa2014 (69.00 ± 2.66) but was significantly longer than in Ekiti2a (66.25 ± 2.66). The average number of plantlets regenerated in Kpamyo (7.50 ± 1.57) was not significantly different from that in Ekiti2a (6.75 ± 1.57) and Asiedu (4.00 ± 1.57), but they were significantly higher than in TDa2014 (2.75 ± 1.57) and Swaswa (1.00 ± 1.57). Asiedu had the highest number of roots formed (6.00 ± 1.05), which was not significantly different from Ekiti2a (5.00 ± 1.05) and Kpamyo (4.50 ± 1.05) but was significantly higher than Swaswa (1.25 ± 1.05) and TDa2014 (2.50 ± 1.05).
Expressed Phytochemicals in Different Yam Genotypes During Somatic Embryogenesis Stages
A total of 27 (12 in callus, 17 in plantlets, and 13 in axillary bud), 22 (7 in callus, 18 in plantlets, and 4 in axillary bud), and 35 (7 in callus, 21 in plantlets, and 13 in axillary bud) phytochemicals were identified in Kpamyo, Ekiti2a, and Asiedu, respectively (Table 3). HC (36.4%, Kpamyo), Tris-tert-butyldimethylsilyloxy-arsane (TRIS: 59.3%, Ekiti2a), and 4-methyl-2-trimethylsililoxy-acetophenone (M42T: 52.7%, Asiedu) were highest in callus. N-Methyl-1-adamantaneacetamide (NM: 31.8%, Kpamyo) and TRIS (52.7%, Ekiti2a, Asiedu) were highest in plantlet regeneration, while TRIS (41.2%, Kpamyo), HC (55.8%, Ekiti2a), and erythro-9,10-dibromopentacosane (38.9%, Asiedu) were highest in axillary bud (Supplementary Information).
In general, out of the phytochemicals identified at the axillary bud stage across the three genotypes, only GA was common to the genotypes. At the callus stage, DT, TRIS, PAA, and HC were found across the three genotypes, while at the PR stage, 13 phytochemicals (TRIS, HC, silicic acid, PAA, methyltris(trimethylsiloxy)silane, 2,4-dimethyl-benzo(h)quinolone (24DB), 2,3-dihydro-6-nitro-1,4-phthalazinedion, 1,2-bis(trimethylsilyl)benzene, HT, 1,4-bis(trimethylsilyl)benzene, 1-methyl-2-phenyl-1H-indole, and 1,2-benziosothiazol-3-amine) were present across the three genotypes.
Relationship Between Identified Phytochemicals and Number of Plantlets Regenerated in Kpamyo, Ekiti2a, and Asiedu (Fig. 4)
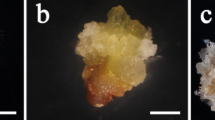
Plantlet regeneration in Dioscorea rotundata through somatic embryogenesis in different media combinations at 10 wk after induction. (A) 4.4 µM benzylaminopurine, 34.0 µM uniconazole-P, and 40.5 µM phenylacetic acid. (B) 4.4 µM benzylaminopurine, 34.0 µM uniconazole-P, and 20.4 µM hexamethylcyclotrisiloxane. (C) 4.4 µM benzylaminopurine, 34 µM uniconazole-P, and 4.8 µM decamethyltetrasiloxane. (D) Hormone-free medium. (E) 4.4 µM benzylaminopurine and 34 µM uniconazole-P. (F) 4.4 µM benzylaminopurine, 34.0 µM UP, and 1.2 µM GLA.
In Kpamyo, decamethyltetrasiloxane, 4-methyl-2-trimethylsililoxy-acetophenone, and methyltris(trimethylsiloxy)silane were significant and positively correlated (r = 1.0) with number of plantlets regenerated. In Ekiti2a, 5-methyl-2-trimethylsilyloxy-acetophenone, hexamethylcyclotrisiloxane, silicic acid, diethyl bis (trimethylsilyl) ester, and 1,2-bis(trimethylsilyl)benzene were positively correlated with plantlet regeneration, while in Asiedu, decamethyltetrasiloxane, arsenous acid, and tris(trimethylsilyl) ester had significant and positive correlation (r = 1.0) with number of plantlets regenerated (Table 4).
Effects of Exogenous Application of Isolated Phytochemicals Expressed by Somatic Embryogenesis–Competent Yam Genotypes on Somatic Embryogenesis of White and Water Yams
The main effects of medium composition on SE were significant. The lowest number of days to callus induction was recorded in the medium without addition of identified phytochemicals so it was the earliest to induce callus (T1: 15.23 ± 3.10). The medium T5 (20.13 ± 1.93) was the latest to induce callus and not significantly different from the conventional multiplication medium, which served as negative control (T6: 19.52 ± 2.51) (Table 5). The same trend was observed for percentage of callus formation, which was highest in T1 (69.00 ± 23.00) while in T5 (18.00 ± 18.00) was lowest. The earliest plantlet regeneration was recorded in M3, in which plantlets were regenerated at an average of 61.20 ± 6.62 d after culture initiation, while it was significantly later in M6 (70.33 ± 4.62) and M2 (65.23 ± 8.17). However, days to plantlet regeneration were not significantly different among M3, M1 (64.4 ± 5.01), M4 (63.40 ± 8.12), and M5 (63.80 ± 6.39) (Table 6). The number of plantlets regenerated was highest in M3 (4.53 ± 0.48), but it was not significantly different from M1 (3.93 ± 0.39). However, they were both significantly higher than the number of plantlets regenerated in other media compositions. The same trend was recorded for number of roots, which was highest in M1 (3.80 ± 0.68) followed by M3 (2.67 ± 0.49) with both being significantly higher than the other treatments.
Table 7 shows that genotypes’ main effect was also significant in response to exogenous application of identified phytochemicals. Kpamyo and Swaswa were latest to form callus (18.88 ± 2.99 and 17.92 ± 3.15 d respectively) relative to other genotypes (lowest = 17.02 ± 3.20 d). The percentage of callus formation was highest in Kpamyo and Ekiti2a (45.00 ± 27.00 and 2.00 ± 26.00 respectively). Table 8 shows that Kpamyo was earliest to regenerate plantlets at 61.50 ± 7.66 d, significantly earlier than other genotypes, the latest being 67.14 ± 6.04 recorded in Asiedu. However, there were no significant differences among the genotypes in the number of plantlets regenerated. The water yam genotypes had the highest number of roots (Swaswa: 2.56 ± 0.61; TDa2014:2.44 ± 0.47) while white yam had the lowest (Asiedu: 1.17 ± 0.36; Ekiti2a: 1.11 ± 0.36; Kpamyo: 1.06 ± 0.41).
Interactions between genotype and medium composition regarding exogenously applied phytochemicals were significant. The days to callus formation ranged from 14 d recorded in Asiedu and Ekiti2a (CIM), Ekiti2a and TDa2014 (CIM plus 28.5 µM PAA), and Swaswa (CIM plus 95.9 µM HC) to 33 d in TDa2014 (CIM plus 95.9 µM HC) (Fig. 2). The percentage callus formation ranged from 7% recorded in Asiedu and Swaswa (CIM plus 9.6 µM DT) to 85% recorded in Ekiti2a (CIM). The numbers of days taken to regenerate yam plantlets ranged from 54 d in PRM plus 1.2 µM GLA (Ekiti2a), PRM plus 4.8 µM DT and PRM plus 20.4 µM HC (Kpamyo), and PRM plus 40.5 µM PAA (TDa2014) to 74 d in YMM (Ekiti2a), PRM plus 1.2 µM GLA (Swaswa), and PRM plus 20.4 µM HC (TDa2014) (Fig. 6). Average number of plantlets regenerated ranged from 0.33 ± 0.58 (PRM plus 20.4 HC in Asiedu) to 7.00 ± 1.73 (PRM plus 40.5 µM PAA in TDa2014) (Fig. 3).
Table 8 shows that the highest callus formation was obtained in medium containing 9.1 µM 2,4-D plus 5.4 µM NAA in all genotypes at baseline and after this study. However, the medium that gave the highest plantlet regeneration varied among genotypes. For Asiedu, Ekiti2a and Swaswa, average of 4.83 plantlets were regenerated from 100%, 7.17 from 86.5%, and 4.70 from 55% of the explants, respectively, on the medium containing 4.4 µM BAP plus 34.0 µM UP. In Kpamyo and TDa2014, medium containing 4.4 µM BAP and 34.0 µM UP plus 40.5 µM PAA had the highest average of 4.30 and 7.00 plantlets regenerated from 82 and 54% of the explants respectively. Comparing the baseline with the realized competence for somatic embryogenesis, the intervention of expressed phytochemicals was higher in water yams than in white yams.
Discussion
The induction of callus is a response of explants to stress, such as wounding and high salt concentration, and is associated with increased formation of ROS at some point during exposure, which may result in the unspecific reduction of proteins and membrane lipids (Joseph and Jini 2010). Similar effects are also achieved through the inclusion of phytochemicals like auxins (2,4-D, NAA, IAA, and picloram) at high concentrations in the culture environment (Momoko et al. 2013). At the callus formation stage, two organosilicons (decamethyltetrasiloxane and hexamethylcyclotrisiloxane) and a carboxylic compound (phenylacetic acid (PAA)) that were identified across the genotypes in this study suggest that they are part of the defense systems to ROS. Amino acids, catalases, peroxidases, and enzymatic scavengers of activated oxygen which can be endogenic in the plants or exogenically applied in the SE culture environment have been reported in other crops (Joseph and Jini 2010).
Organosilicons are linked to antioxidant activities that are essential in plant defense mechanism in response to stress (Ethela et al. 2017), while PAA, being a naturally occurring auxin, has been used to maintain callus proliferation in tobacco, sunflower, and pea although at higher concentrations of 25 to 500 µM (Leuba and Letourneau 1990). Organosilicons have antioxidant properties, as demonstrated in Origanum vulgare (Galehassadi et al. 2014), and silicon-derived amino acids have also been described as a potent antioxidant (Annaliese and Sean 2013). Antioxidants are essential in callus cultures to reduce ROS (Momoko et al. 2013) because too much oxidation can cause the callus to turn brown and eventually die back. However, the optimum concentration and the time of exogenous application during callus induction are yet to be determined in order to avoid its being counterproductive in the case of SE since the interest is to produce more callus.
More phytochemicals were identified at the plantlet regeneration stage while the callus induction stage had the least number of phytochemicals. This could be that the plantlet regeneration stage is more complex, requiring more phytochemicals or antioxidants for the conversion of somatic embryos into plants. This finding could be an insight to the recommendation of Gaj (2004) for the hormonal characterization of somatic embryogenesis environment and responsive cells in plants. In the axillary bud explant used, out of the 26 phytochemicals discovered, only glutaric acid belonging to the carboxylic group of compounds was identified across the three genotypes. Glutaric acid is a dicarboxylate analog of glutamic acid, and aspartic acids are amino acids that have been reported in yam (Doss et al. 2019). Other phytochemicals belonging to the carboxylic group are dl-aspartic acid and phenylacetic acid. Aspartic acid is a precursor of four essential amino acids: asparagine, methionine, threonine, and lysine (Johnson 2017), and both glutaric acid and aspartic acid have been shown to have significant positive correlation with the sprouting of Saffron corm (Bagri et al. 2017), which is a vegetative propagative part of Saffron like the axillary buds of in vitro yam plantlets. Another carboxylic compound identified in the axillary bud explant of yam in this study is l-tryptophan, an essential plant-derived amino acid needed for the in vivo biosynthesis of proteins (Mendel 2018) and a precursor for the production of indole-3-acetic acid (Lalit 2002) which is an auxin that plays a role in callus formation (Harish et al. 2010). The incorporation of tryptophan in callus induction and plantlet-regenerating media of rice favored callus induction and plantlet regeneration through somatic embryogenesis (Shahsavari 2011).
The addition of the identified phytochemicals to callus induction medium successfully induced calluses in the different genotypes within a 3-wk period, except for the addition of decamethyltetrasiloxane to callus induction medium that could not induce callus in TDa2014. A higher or lower concentration than what was applied could have produced the desired effect if its stability varies significantly with environment (Mathe et al. 2012). However, this was not significantly different from the medium without the phytochemicals. The baseline callus induction medium gave the highest percentage callus induction (69%) in all genotypes except Swaswa where addition of glutaric acid to callus induction medium improved callus formation. Callus induction from the treatments containing the two (2) organosilicons (decamethyltetrasiloxane and hexamethylcyclotrisiloxane) was less than the 22% induction rate from the PGR-free yam multiplication medium, probably due to habituation (Ossai 2014). Studies have shown that an appropriate combination of NAA and 2,4-D gave the best condition for callus induction in yams (Belarmino and Gonzales 2008; Ossai et al. 2018). Both decamethyltetrasiloxane and hexamethylcyclotrisiloxane as detected from the callus explants of Asiedu, Ekiti2a, and Kpamyo might not be playing an active role in callus formation but on the protection of the plants from free radicals (Galehassadi et al. 2014). This can be confirmed using more specific methods like radioactive labeling (Khosrav et al. 2019).
The Dioscorea alata genotypes responded more favorably than the Dioscorea rotundata genotypes in terms of gain in SE from baseline to the realized plantlet regeneration. This could be that the D. alata genotypes respond more or are more affected by the changes in environmental conditions. Fluctuations in the concentration of endogenous hormones in water yam were reported to be high (Matsumoto et al. 2013). The white yams on the other hand are more stable, thereby having little changes in SE protocol alterations. Despite the superior effect of baseline callus induction medium on callus formation relative to others, addition of 2.1 µM GLA gave above 50% callus induction rate in the three (3) genotypes of white yam. Although there is a dearth of information on the use of glutaric acid to induce callus in yam, the application of ketoglutaric acid at 100 mgL−1 was reported to improve the formation of embryogenic callus from Zoysia japonica seeds (Asano et al. 1996).
The use of 4.4 µM BAP plus 34.0 µM UP for plantlet regeneration had been reported by Ossai et al (2018) as the best treatment combination for plantlet regeneration in yam through somatic embryogenesis. In this study, an addition of 40.5 µM PAA to PRM also gave improved plantlet regeneration from the somatic embryos of Kpamyo and TDa2014 by 5.39% and 343.04%, respectively. It also supported better root formation from the somatic embryos of the five (5) yam genotypes than other treatments. Phenylacetic acid has been used in the direct regeneration of chickpea (Ghanti et al. 2009). Auxins like phenylacetic acid at high concentrations in culture media are mostly used to induce callus by causing an oxidative stress to the wounded explants. However, PAA has been reported to possess moderate levels of free radical scavenging activities in Ilex aquifolium (Nahar et al. 2005; Kazan and Manners 2009). Akram et al. (2016) also found that PAA elicits induced systemic resistance (IRS: resistance mechanism in plants activated by infection or injuries) in plants, which is accompanied by the expression of defense-related genes and proteins (Zamioudis and Pieterse 2012).
Conclusion
A total of 27, 22, and 35 different phytochemicals were identified in callus, axillary buds, and plantlets of white and water yam. Out of the tested exogenously, phenylacetic acid improved plantlet regeneration significantly while decamethyltetrasiloxane and hexamethylcyclotrisiloxane were detrimental. The implications of these to the seed system development are the need for further standardization of the timing of application and different concentrations of phytochemicals towards the adoption of the SE system. This is expected to lead to significant increase in the multiplication ratio of yam leading to its availability all season. However, it is necessary to confirm the genetic integrity of the plantlets regenerated since somaclonal variation is indispensable during SEs.
Data Availability
All data used during the study are available from the author Ossai Chukwunalu Okolie by request (c.ossai@cgiar.org).
References
Adeigbe OO, Ilori CO, Adewale BD (2015) Phenotypic diversity and ploidy level of some Dioscorea dumetorum genotypes. J Agr Veterin Sci 8:47–52
Ahmad P, Abdel L, Arafat AH, Rasool S, Akram NA, Ashraf M, Gucel S (2016) Role of proteomics in crop stress tolerance. Front Plant Sci 7:1337. https://doi.org/10.3389/fpls.2016.01336
Akram W, Anjum T, Ali B (2016) Phenylacetic acid is ISR determinant produced by Bacillus fortis IAGS162, which involves extensive re-modulation in metabolomics of tomato to protect against Fusarium wilt. Front Plant Sci 7:89–98
Annaliese KF, Sean OW (2013) Organosilicon molecules with medicinal applications. J Med Chem 56:388–405
Asano Y, Katsumoto H, Inokuma C, Kaneko S, Ito Y, Fujiie A (1996) Cytokinin and thiamine requirements and stimulative effects of riboflavin and ketoglutaric acid on embryogenic callus induction from the seeds of Zoysia japonica Steud. J Plant Physio 149:413–417
Bagri J, Yadav A, Anwar K, Dkhar J, Singla-Pareek SL, Pareek A (2017) Metabolic shift in sugars and amino acids regulates sprouting in Saffron corm. Sci Rep 7:17–28
Balogun MO, Maroya N, Aighewi B, Mignouna D (2021) Manual for seed yam production in hydroponics system. International Institute of Tropical Agriculture (IITA). 31 pp
Balogun MO, Maroya N, Asiedu R (2014) Status and prospects for improving yam seed systems using temporary immersion bioreactors. Academic J 13:1614–1622
Balogun MO, Maroya N, Taiwo J, Ossai C, Ajayi A, Kumar PL, Pelemo O, Aighewi B, Asiedu R (2017) Clean seed yam tuber production using temporary immersion bioreactors. International Institute of Tropical Agriculture (IITA) p 66
Belarmino MM, Gonzales JR (2008) Somatic embryogenesis and plant regeneration in purple food yam (D. alata L.). Ann Trop Res 30:22–33
Bradford MM (1976) A rapid and sensitive method for quantification of microgram qualities of protein, utilizing the principle of protein-dry binding. Anal Biochem 72:248–254
Coyne DL, Claudius-Cole AO, Kenyon L, Baimey H (2009) Differential effect of hot water treatment on whole tubers versus cut setts of yam (Dioscorea spp.). Pest Mgt Sci 10:81–87
Doss A, Tresina PS, Mohan VR (2019) Amino acid composition of wild yam (Dioscorea spp.). Food Res 3:617–621
Ethela W, Ana TF, Mikaila PP, Angelica SDR, Ane GV, Andre LS, Gilson Z, Cristiane L (2017) Antioxidant effect of quinoline derivatives containing or not selenium: relationship with antinoceptive action quinolines are antioxidant and antinociceptive. An Braz Acad Sci 89:457–467
Gaj MD (2004) Factors influencing somatic embryogenesis and plant regeneration with particular reference to Arabidopsis thaliana (L.) Heynh. Plant Cell Tiss Org Cultu 43:27–27
Galehassadi M, Razai E, Najarand S, Mahkam M, Mohammadzadeh GN (2014) Isolation of carvacol from Origanum vulgare, synthesis of some organosilicon derivatives, and investigating of its antioxidant, antibacterial activities. Standard Sci Res Essays 2:438–450
Ghanti SK, Sujata KG, Rao MS (2009) The effect of phenylacetic acid on shoot bud induction, elongation and rooting of Chickpea. Biol Plantar 53:779–783
Graf G, Ben-Meir H, Avivi Y, Moshe M, Dahan Y, Zemach A (2007) Histone methylation controls telomerase-independent telomere lengthening in cells undergoing dedifferentiation. Dev Biol 306:838–846
Harish MC, Rajeevkumar S, Sathishkumar R (2010) Efficient in vitro callus induction and regeneration of different tomato cultivars of India. Asian J Biotech 2:178–184
Johnson EC (2017) Aspartic acid. Reference Module in Biomedical Sciences. Elsevier pp 1–4
Joseph B, Jini D (2010) Insight into the role of antioxidant enzymes for salt tolerance in plants. Int J Bot 6:456–464
Junaid A, Srivastava PS, Sharma MP (2013) Somatic embryogenesis and gene expression, Edition: 1st Chapter: Factors regulating somatic embryogenesis in plants. Narosa Publishing House, New Delhi pp 56:81
Kakkar RK, Sawhney VK (2002) Polyamines research in plants – a changing perspective. Plant Physiol 116:281–292
Kazan K, Manners JM (2009) Linking development to defense: auxin in plant–pathogen interactions. Trends Plant Sci 14:373–382
Khosrav P, Heidari-Soureshjani S, Yang Q (2019) Effects of medicinal plants on radiolabeling of diagnostic radiopharmaceuticals: a review. Plant Sci Today 6:123–131
Lalit MS (2002) Plant growth and development: hormones and environment. Auxins. Acad Press p 155–169
Leuba V, Letourneau D (1990) Auxin activity of phenylacetic acid in tissue culture. J Plant Growth Reg 9:71–76
Loretta G (2014) Plant phenotypic plasticity in response to environmental factors. Adv Bot 2014:1–17
Manoharan R, Tripathi JN, Tripathi L (2016) Plant regeneration from axillary bud derived callus in white yam (Dioscorea rotundata). Plant Cell Tiss Org Cult 126:481–488
Maroya N, Balogun M, Asiedu R, Aighewi B, Kumar PL, Augusto J (2014) Yam propagation using aeroponics technology. Ann Res Rev Biol 4:3894–3903
Mathe C, Mosolygo A, Suranyi G, Beke A, Demeter Z, Toth VR (2012) Genotype and explant type dependent morphogenesis and silicon response of common reed (Phragmites australis) tissue cultures. Aquat Bot 97:57–63
Matsumoto R, Kikuno H, Shiwachi H, Toyohara H, Takebayashi Y, Jikumaru Y, Kamiya Y (2013) Growth of vine cuttings and fluctuations of concentrations of endogenous plant hormones in water yam (Dioscorea alata L.). Trop Agric Dev 57:23–30
Mendel F (2018) Analysis, nutrition, and health benefits of tryptophan. Int J Tryptophan Res 11:1–12
Momoko I, Keiko S, Akira I (2013) Plant callus: mechanisms of induction and repression. Plant Cell pp 1–5
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–492
Nahar L, Russel WR, Middleton M, Shoeb M, Sarkers SD (2005) Antioxidant phenylacetic acid derivatives from the seeds of Ilex aquifolium. Acta Pharm 55:187–193
Ossai C, Balogun M, Maroya N, Asiedu R (2018) Development of micropropagation system for yam (Dioscorea spp.) using somatic embryogenesis. YIIFSWA Research Brief, Int Inst. Trop. Agric, pp 1–7
Ossai CO (2014) Development of protocols for somatic embryogenesis yams (Dioscorea spp) towards scale up in temporary immersion bioreactor system (TIBs). M.Sc. Thesis, University of Ibadan pp 76
Polycarp D, Afoakwa EO, Budu A, Otoo E (2012) Characterization of chemical composition and anti-nutrition factors in seven species within the Ghanaian yam (Dioscorea) germplasm. Int Food Res J 19:985–992
Saleethong P, Roytrakul S, Kong-Ngern K, Theerakulpisut P (2016) Differential proteins expressed in rice leaves and grains in response to salinity and exogenous spermidine treatments. Rice Sci 23:9–21
Santos IR, Maximiano MR, Almeida RF, da Cunha RNV, Lopes R, Scherwinski-Pereira JE, Mehta A (2018) Genotype-dependent changes of gene expression during somatic embryogenesis in oil palm hybrids (Elaeis oleifera x E. guineensis). PLoS ONE 13:209–214
Shahsavari E (2011) Impact of tryptophan and glutamine on tissue culture of upland rice. Plant Soil Env 57:7–10
Zamioudis C, Pieterse CM (2012) Modulation of host immunity by beneficial microbes. Mol Plant Microbe Interact 25:139–150
Acknowledgements
This research was supported by the Bill and Melinda Gates Foundation through the YIIFSWA II project of IITA, Ibadan. The authors would like to thank Professor Iyiola Fawole, Dr. Badara Gueye, and Late Dr. Christopher Ilori for their professional guidance.
Funding
This work was supported by the Bill and Melinda Gates Foundation (OPP1159088).
Author information
Authors and Affiliations
Contributions
Conceptualization: C.O.O., M.O.B., M.A.S., and N.G.M. Data curation: C.O.O. Methodology: C.O.O., M.O.B., M.A.S., and N.G.M. Formal analysis: C.O.O. Writing—original draft: C.O.O. Writing—review and editing: C.O.O., M.O.B., N.G.M., and M.A.S.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ossai, C.O., Balogun, M.O., Maroya, N.G. et al. Quantification of endogenous phytochemicals and determination of their exogenous effects in somatic embryogenesis pathways of white and water yams. In Vitro Cell.Dev.Biol.-Plant 59, 29–38 (2023). https://doi.org/10.1007/s11627-023-10337-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11627-023-10337-5