Abstract
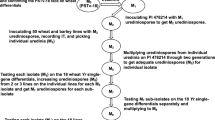
The development of Dendrobium ‘Earsakul’ with improved resistance to black rot is essential for sustainable orchid production. In this study, in vitro mutagenesis and selection was used to breed D. ‘Earsakul’ for black rot resistance, evaluated black rot resistance levels in mutants and controls using detached leaf assay, and characterized putative resistant mutants using molecular markers, cytology, and morphological traits. Mutagenized protocorm-like bodies (PLBs) obtained by 1.4% (LD30) and 1.8% (LD50) ethyl methanesulfonate (EMS) concentrations and non-mutagenized PLBs were selected on a pea sucrose broth (PSB) medium supplemented with 0, 30, and 50% (first 2 cycles) and 0, 40, and 60% (third cycle) of Phytophthora palmivora culture filtrates (CFs). Fifty putative resistant mutants were obtained, and 42 of these were used for resistance level evaluation by detached leaf assay using P. palmivora isolate NK-53–9. The results revealed 13 resistant putative mutants, including 4 highly resistant putative mutants and 9 resistant putative mutants. Ten surviving resistant putative mutants were genetically different from the non-mutagenized controls and were confirmed as mutants after inter-simple sequence repeat (ISSR) analysis. Based on flow cytometry, the resistant mutants and non-mutagenized controls possessed the same chromosome number of 2n + 4n + 8n. Moreover, a mutant, SUT17E18316, exhibiting maximum DNA content and genome size was identified. Morphological characterization revealed that most of the black rot–resistant mutants were morphologically different on some characters from non-mutagenized lines, such as plant height and number of roots. Particularly, the highly resistant mutant SUT13E18305 which possessed outstanding characters may be useful for future commercialization.








Similar content being viewed by others
References
Abeyawardana OAJ, Koudela M (2019) In vitro selection for Fusarium oxysporum f. sp. conglutinans resistance in Brassica vegetables. Int J Sci Rep 5:35–44
Al-Qurainy F, Khan S (2009) Mutagenic effects of sodium azide and its application in crop improvement. World Appl Sci J 6:1589–1601
Arisha MH, Liang BK, Shah SNM, Gong ZH, Li DW (2014) Kill curve analysis and response of first generation Capsicum annuum L. B12 cultivar to ethyl methanesulfonate. Genet Mol Res 13:10049–10061
Atichart P (2013) Polyploid induction by colchicine treatments and plant regeneration of Dendrobium chrysotoxum. Thai J Agric Sci 46:59–63
Baillieul F, de Ruffray P, Kauffmann S (2003) Molecular cloning and biological activity of α-, β-, and γ-megaspermin, three elicitins secreted by Phytophthora megasperma H20. Plant Physiol 131:155–166
Bennett MD, Smith JB (1976) Nuclear DNA amounts in angiosperms. Philos Trans R Soc Lond 274:227–274
Behera M, Panigrahi J, Mishra RR, Rath SP (2012) Analysis of EMS induced in in vitro mutants of Asteracantha longifolia (L.) Nees using RAPD markers. Indian J Biotechnol 11:39–47
Capasso R, Cristinzio G, Di Maro A, Ferranti P, Parente A (2001) Syringicin, a new α-elicitin from an isolate of Phytophthora syringae, pathogenic to citrus fruit. Phytochemistry 58:257–262
Cating RA, Palmateer AJ, Stiles CM, Rayside PA (2010) Black rot of orchids caused by Phytophthora cactorum and Phytophthora palmivora in Florida. Plant Health Prog 11:39–43
Constantin MJ (1984) Potential of in vitro mutation breeding for the improvement of vegetatively propagated crop plants. In: Proceedings of the International Atomic Energy Agency (IAEA). Vienna, Austria, pp 59–77
De LC, Rao AN, Rajeevan PK, Srivastava M, Chhetri G (2015) Morphological characterization in Dendrobium species. Journal of global Biosciences 4:1198–1215 https://www.researchgate.net/publication/335443240
Dong S, Kong G, Qutob D, Yu X, Tang J, Kang J, Dai T, Wang H, Gijzen M, Wang Y (2012) The NLP toxin family in Phytophthora sojae includes rapidly evolving groups that lack necrosis-inducing activity. Mol Plant Microbe Interact 25:896–909
Du J, Verzaux E, Chaparro-Garcia A, Bijsterbosch G, Keizer LC, Zhou J, Liebrand TWH, Xie C, Govers F, Robatzek S, van der Vossen EAG, Jacobsen E, Visser RGF, Kamoun S, Vleeshouwers VG (2015) Elicitin recognition confers enhanced resistance to Phytophthora infestans in potato. Nat Plants 1:15034
El-Kazzaz AA, Ashour AMA (2004) Genetically resistant cucumber plants to wilt pathogen via tissue cultures. Egypt J Phytopathol 32:1–10 http://www.academia.edu/27396951
El-Kazzaz AA, Hanafy MS, Abdel-Kader MM (2009) In vitro selection of resistant rice plants against rice blast caused by Pyricularia oryzae via tissue culture technique. Arch Phytopathol Plant Prot 42:847–856. https://doi.org/10.1080/03235400701492715
Hofmann NE, Raja R, Nelson RL, Korban SS (2004) Mutagenesis of embryogenic cultures of soybean and detecting polymorphisms using RAPD markers. Biol Plant 48:173–177
Hualsawat S (2019) Characterization of sodium azide induced black rot resistant Dendrobium ‘Earsakul’ mutants. MSc Thesis. School of Crop Production Technology, Suranaree University of Technology, Nakhon Ratchasima http://sutir.sut.ac.th:8080/jspui/handle-/123456789/8572
Keim P, Beavis W, Schupp J, Freestone R (1992) Evaluation of soybean RFLP marker diversity in adapted germ plasm. Theor Appl Genet 85:205–212
Khairum A, Tharapreuksapong A, Wongkaew S, Tantasawat PA (2016). Cultural characteristics and pathogenicity analysis of Phytophthora palmivora, causal pathogen of black rot in orchids. In: Rahman MA, Dasic P (eds) Proc Int Confer Agric Food Biol Health Sci (AFBHS-16). Kuala Lumpur, Malaysia, pp 101–106
Khairum A, Poolsawat O, Pornbungkerd P, Tharapreuksapong A, Wongkaew S, Tantasawat PA (2018) Effects of culture media on Phytophthora palmivora growth, α-elicitin production and toxicity to Dendrobium. Not Bot Horti Agrobot Cluj Napoca 46:630–638
Lerthiran P (2020) Orchid products. Department of International Trade Promotion. https://ditp.go.th/ditp_web61/article_sub_view.php?filename=contents_attach/730251/730251.pdf&title=730251&cate=743&d=0. Cited 26 May 2021
Levesque R, Inc SPSS (2006) SPSS programming and data management, 3rd edn. SPSS Institute, New York
Malik NAA, Kumar IS, Nadarajah K (2020) Elicitor and receptor molecules: orchestrators of plant defense and immunity. Int J Mol Sci 21:963–996
Mantel N (1967) The detection of disease clustering and a generalized regression approach. Cancer Res 27:209–220
Morris SM, Domon OE, McGarrity LJ, Kodelo RL, Casciano DA (1992) Effect of bromodeoxyuridine on the proliferation and growth of ethyl methanesulfonate-exposed P3 cells: relationship to the induction of sister-chromatid exchanges. Cell Biol Toxicol 8:75–87
Muangsorn S, Te-chato S (2008) Use of EMS to induce mutation in Dendrobium friedericksianum Rchb.f. J Agriculture 24:153–164 https://li01.tci-thaijo.org/index.php/joacmu/article/view/246286/168404
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Nyassé S, Cilas C, Herail C, Blaha G (1995) Leaf inoculation as an early screening test for cocoa (Theobroma cacao L.) resistance to Phytophthora black pod disease. Crop Prot 14:657–663
Pettongkhao S, Navet N, Schornack S, Tian M, Churngchow N (2020) A secreted protein of 15 kDa plays an important role in Phytophthora palmivora development and pathogenicity. Sci Rep 10:2319–2333
Rohlf FJ (1998) NTSYSpc numerical taxonomy and multivariate analysis system version 2.2 user guide. Appl Biostat Inc, New York
Ryu J, Ha BK, Kim DS, Kim JB, Sangjin K, Ahn JW, Jeong IY, Jo HJ, Kim EY, Kang SY (2014) Genetic diversity and variation analysis of mutant lines derived from γ-ray and chemical mutagen treatments in blackberry (Rubus fruticosus). Plant Genet Res 12:S114–S117
Scala A (1989) Toxins produced in culture by Phytophthora infestans race Tl and some of their effects on tomato. In: Graniti A, Durbin RD, Ballio A (eds) Phytotoxins and plant pathogenesis. Springer, Berlin, pp 453–454
Samala S, Te-chato S, Yenchon S (2014) Effect of ethyl methanesulphonate (EMS) on Dendrobium Sonia. Khon Kaen Agri J 42:506–511
Sambrook J, Russell DW (2001) Molecular cloning: a laboratory manual, 3rd edn. Cold Spring Harbor Laboratory Press, New York, A9.5–A9.7
Sartori A, Bevilacqua D, Terlizzi M, Di Cintio A, Rosato T, Caboni E, Ferrante P, Scortichini M, Cipriani G (2015) EMS mutagenesis and selection of genotypes resistant or tolerant to Pseudomonas syringae pv. actinidiae. Acta Hortic 1096:221–228
Sari DR, Aloysius S (2021) Genetic diversity of Spathoglottis plicata Blume orchid variant based on inter-simple sequence repeat (ISSR) molecular marker. ASSEHR 528:62–69
Savita VGS, Nagpal A (2011) In vitro selection of calli of Citrus jambhiri Lush. for tolerance to culture filtrate of Phytophthora parasitica and their regeneration. Physiol Mol Biol Plants 17:41–47
Savita PP, Virk G, Nagpal A (2017) In vitro selection of resistant/tolerant mutants lines of Citrus jambhiri Lush. using crude culture filtrate of Phytophthora parasitica and their randomly amplified polymorphic DNA analysis. J Phytopathol 165:771–781
Saubeau G, Gaillard F, Legentil L, Nugier-Chauvin C, Ferrières V, Andrivon D, Val F (2014) Identification of three elicitins and a galactan-based complex polysaccharide from a concentrated culture filtrate of Phytophthora infestans efficient against Pectobacterium atrosepticum. Molecules 19:15374–15390
Smith JSC, Chin ECL, Shu H, Smith OS, Wall SJ, Senior ML, Mitchell SE, Kresovich S, Ziegle J (1997) An evaluation of the utility of SSR loci as molecular markers in maize (Zea mays L.): comparisons with data from RFLPs and pedigree. Theor Appl Genet 95:163–173
Tantasawat PA, Khairum A, Arsakit K, Poolsawat O, Pornbungkerd P, Kativat C (2015) Effect of different culture media on growth and proliferation of Dendrobium ‘Earsakul’ protocorm-like body. HortTechnology 25:681–686
Tantasawat TP, Khairum A, Tharapreuksapong A, Poolsawat O, Tantasawat PA (2017) Molecular characterization of Dendrobium ‘Earsakul’ mutants from in vitro selection for black rot resistance. J Appl Hortic 19:130–134
The Government Public Relations Department (2018) Thailand recognized as the world’s largest producer of tropical orchids. https://thailand.prd.go.th/ewt_news.php?nid=6263 &filename=index. Cied 26 May 2021
Tignon M, Kettmann R, Watillon B (2002) AFLP: use for the identification of apple cultivars and mutants. Acta Hortic 521:219–226
Till BJ, Cooper J, Tai TH, Colowit P, Greene EA, Henikoff S, Comai L (2007) Discovery of chemically induced mutations in rice by TILLING. BMC Plant Biol 7:19–30
Till BJ, Reynolds SH, Weil C, Springer N, Burtner C, Young K, Bowers E, Codomo CA, Enns LC, Odden AR, Greene EA, Comai L, Henikoff S (2004) Discovery of induced point mutations in maize genes by TILLING. BMC Plant Biol 4:12–19
Uchida JY (1994) Diseases of orchids in Hawaii. Plant Dis 78:220–224
Vacin EF, Went FW (1949) Some pH changes in nutrient solution. Bot Gaz 110:605–613
Val F, Desender S, Bernard K, Potin P, Hamelin G, Andrivon D (2008) A culture filtrate of Phytophthora infestans primes defense reaction in potato cell suspensions. Phytopathology 98:653–658
van Harten AM (1998) Mutation breeding: theory and practical applications. Cambridge University Press, Cambridge
Velmurugan M, Rajamani K, Paramaguru P, Gnanam R, Kannan Bapu JR, Harisudan C, Hemalatha P (2010) In vitro mutation in horticultural crops - a review. Agric Rev 31:63–67
Wannajindaporn A, Poolsawat O, Chaowiset W, Tantasawat PA (2014) Evaluation of genetic variability in in vitro sodium azide-induced Dendrobium ‘Earsakul’ mutants. Genet Mol Res 13:5333–5342
Wannajindaporn A, Kativat C, Tantasawat PA (2016) Mutation induction of Dendrobium ‘Earsakul’ using sodium azide. HortScience 51:1363–1370
Wu L, Li M, Yang X, Yang T, Wang J (2011) ISSR analysis of Chlorophytum treated by three kinds of chemical mutagen. J Northeast Agric Univ 18:21–25
Yoosumran V, Ruamrungsri S, Duangkongsan W, Kanjana S (2018) Induced mutation of Dendranthemum grandiflora through tissue culture by ethyl methanesulphonate (EMS). IJAT 14:73–82
Zhang MB, Pan LJ, Fan GQ, Chen JM, Cheng P (2009) Study on DNA isolation from polysaccharides-rich transgenic Dendrobium. Mol Plant Breed 7:209–214
Acknowledgements
This work was supported by Suranaree University of Technology (SUT) and by Thailand Science Research and Innovation (TSRI). The authors would also like to acknowledge Ministry of Education and grants from National Research Council of Thailand (NRCT). We are very grateful to Peter C. Bint for editing the manuscript.
Author information
Authors and Affiliations
Contributions
PAT designed and guided the experiments. AK, PAT, WC, SH, AT, BT, SY, and PP performed the experiments, analyzed the data, wrote the article, and revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Rights and permissions
About this article
Cite this article
Khairum, A., Hualsawat, S., Chueakhunthod, W. et al. Selection and characterization of in vitro–induced mutants of Dendrobium ‘Earsakul’ resistant to black rot. In Vitro Cell.Dev.Biol.-Plant 58, 577–592 (2022). https://doi.org/10.1007/s11627-022-10261-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11627-022-10261-0