Abstract
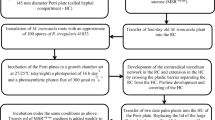
In vitro cultivation systems of arbuscular mycorrhizal fungi are useful tools to study the interaction between plants and their fungal symbiont, and also to develop new biotechnologies. Plantlets of the latex-producing species Hevea brasiliensis clone PB 260 were grown in a dense extraradical mycelium network of the arbuscular mycorrhizal fungus Rhizophagus irregularis MUCL 41833 developed from a mycelium donor plant (Medicago truncatula A17). The factors indole-3-butyric acid (IBA), 2-morpholineoethanesulfonic acid monohydrate (MES) buffer, and carbon dioxide (CO2) were tested on root development and colonization by the fungus. No colonization was observed in the presence of plantlets pre-treated with IBA. The highest levels of root colonization were obtained when plantlets were mycorrhized under a high CO2 concentration (1,000 μmol mol−1) with MES (10 mM) added to the growth medium. Widespread root colonization (with presence of arbuscules, intraradical mycelium, and spores/vesicles) was predominantly observed in newly produced roots. Therefore, it appears essential to improve root initiation and growth for improving in vitro mycorrhization of H. brasiliensis. We demonstrated the potential of the “mycelium donor plant” in vitro culture system to produce colonized H. brasiliensis plantlets before their transfer to ex vitro conditions.


Similar content being viewed by others
References
Becard G, Douds DD, Pfeffer PE (1992) Extensive in vitro hyphal growth of vesicular-arbuscular mycorrhizal fungi in the presence of CO2 and flavonols. Appl Environ Microbiol 58:821–825
Becard G, Piche Y (1989) Fungal growth stimulation by CO2 and root exudates in vesicular–arbuscular mycorrhizal symbiosis. Appl Environ Microbiol 55:2320–2325
Blanc G, Michaux-Ferrière N, Teisson C, Lardet L, Carron MP (1999) Effects of carbohydrate addition on the induction of somatic embryogenesis in Hevea brasiliensis. Plant Cell Tiss Organ Cult 59:103–112
Buddendorf-Joosten JMC, Woltering EJ (1994) Components of the gaseous environment and their effects on plant growth and development in vitro. Plant Growth Regul 15:1–16
Carron MP, Campagna S, Chaine C, Etienne H, Lardet L, Leconte A (1995) Somatic embryogenesis in rubber (Hevea brasiliensis Müll. Arg.). In: Jain SM, Gupta PK, Newton RJ (eds) Somatic embryogenesis in woody plants. Kluwer Academic Publishers, Dordrecht, pp 117–136
Carron MP, Lardet L, Leconte A, Boko C, Dea BG, Keli J (2003) Field growth and rubber yield of Hevea brasiliensis (Muëll. -Arg.) from budded versus in vitro micropropagated plants from clone IRCA 18. Acta Hortic 616:283–293
Carron MP, Lardet L, Leconte A, Dea BG, Keli J, Granet F, Julien J, Teerawatanasuk K, Montoro P (2009) Field trials network emphasizes the improvement of growth and yield through micropropagation in rubber tree (Hevea brasiliensis, Muëll.-Arg.). Acta Hortic 812:485–492
Compagnon P, D’Auzac J (1986) Le Caoutchouc naturel: Biologie, culture, production. G.-P. Maisonneuve et Larose, Paris
Correa M, Martinez J, Montenegro J (1993) Análisis sobre la actividad de hongos formadores de micorrizas vesiculo arbusculares. In: Saldarriaga JG, van der Hammen T (eds) Aspectos ambientales para el ordenamiento territorial del occidente del departamento del Caquetá. IGAG, Bogotá, pp 698–736
Cranenbrouck S, Voets L, Bivort C, Renard L, Stullu DG, Declerck S (2005) Methodologies for in vitro cultivation of arbuscular mycorrhizal fungi with root organs. In: Declerck S, Strullu DG, Fortin JA (eds) In vitro culture of mycorrhizas. Springer, Berlin, pp 341–348
de Klerk GJ, van der Krieken W, de Jong J (1999) Review the formation of adventitious roots: new concepts, new possibilities in vitro. Cell Dev Biol Plant 35:189–199
Declerck S, Strullu DG, Plenchette C (1998) Monoxenic culture of the intraradical forms of Glomus sp. isolated from a tropical ecosystem: a proposed methodology for germplasm collection. Mycologia 90:579–585
Delabarre MA, Serier JB (1995) L’Hévéa. Maisonneuve et Larose, Paris
Díaz-Pérez JC, Sutter EG, Shackel KA (1995) Acclimatization and subsequent gas exchange, water relations, survival and growth of microcultured apple plantlets after transplanting them in soil. Physiol Plant 95:225–232
Díez J, Manjón JL, Kovács GM, Celestino C, Toribio M (2000) Mycorrhization of vitroplants raised from somatic embryos of cork oak (Quercus suber L.). Appl Soil Ecol 15:119–123
Douds DD Jr (1997) A procedure for the establishment of Glomus mosseae in dual culture with Ri T-DNA-transformed carrot roots. Mycorrhiza 7:57–61
Dupré de Boulois H, Voets L, Declerck S (2009) In vitro compartmented systems to study transport in arbuscular mycorrhizal symbiosis. In: Varma A, Kharkwal A (eds) Symbiotic fungi. Springer, Berlin, pp 101–122
Elmeskaoui A, Damont JP, Poulin MJ, Piche Y, Desjardin Y (1995) A tripartite culture system for endomycorrizal inoculation of micropropagated strawberry plantlets in vitro. Mycorrhiza 5:313–319
Fabbri A, Bartolini G, Lambardi M, Kailis S (2004) Olive propagation manual. Landlinks, Collingwood
Fitter AH, Heinemeyer A, Staddon PL (2000) The impact of elevated CO2 and global climate change on arbuscular mycorrhizas: a mycocentric approach. New Phytol 147:179–187
Fujiwara K, Kozai T (1995) Physical microenvironment and its effects. In: Aitken-Christie J, Kozai T, Smith MAL (eds) Automation and environmental control in plant tissue culture. Kluwer Academic Publishers, Dordrecht, pp 319–369
Gallou A, De Jaeger N, Cranenbrouck S, Declerck S (2010) Fast track in vitro mycorrhization of potato plantlets allow studies on gene expression dynamics. Mycorrhiza 20:201–207
Gryndler M, Hršelová H, Chvátalová I, Vosátkaand M (1998) In vitro proliferation of Glomus fistulosum intraradical hyphae from mycorrhizal root segments of maize. Mycol Res 102:1067–1073
Harbage JF, Stimart DP (1996) Effect of pH and 1H-indole-3-butyric acid (IBA) on rooting of apple microcuttings. J Am Soc Hortic Sci 121:1049–1053
Hazarika BN (2006) Morpho-physiological disorders in in vitro culture of plants. Sci Hortic 108:105–120
Ikram A, Mahmud A, Ghani M, Ibrahim M, Zainal A (1992) Field nursery inoculation of Hevea brasiliensis Muell. Arg. seedling rootstock with vesicular–arbuscular mycorrhizal (VAM) fungi. Plant Soil 145:231–236
Ikram A, Mahmud AW, Othman H (1993) Growth response of Hevea brasiliensis seedlings rootstock to inoculation with vesicular–arbuscular mycorrhizal fungal species in steam-sterilised soil. J Nat Rubber Res 8:231–242
Jeong RB, Fujiwara K, Kozai T (1995) Environmental control and photoautotropic micropropagation. In: Janick J (ed) Horticultural reviews, vol 17. Wiley, New York, pp 125–172
Kaldorfa M, Ludwig-Muller J (2000) AM fungi might affect the root morphology of maize by increasing indole-3-butyric acid biosynthesis. Physiol Plant 109:58–67
Karandashov V, Kuzovkina I, Hawkins HJ, George E (2000) Growth and sporulation of the arbuscular mycorrhizal fungus Glomus caledonium in dual culture with transformed carrot roots. Mycorrhiza 10:23–28
Koffi MC, de la Providencia IE, Elsen A, Declerck S (2009) Development of an in vitro culture system adapted to banana mycorrhization. Afr J Biotechnol 8:2750–2756
Koffi MC, Vos C, Draye X, Declerck S (2012) Effects of Rhizophagus irregularis MUCL 41833 on the reproduction of Radopholus similis in banana plantlets grown under in vitro culture conditions. Mycorrhiza. doi:10.1007/s00572-012-0467-6
Kozai T, Kubota C (2001) Developing a photoautotrophic micropropagation system for woody plants. J Plant Res 114:525–537
Lardet L, Martin F, Dessailly F, Carron MP, Montoro P (2007) Effect of exogenous calcium on post-thaw growth recovery and subsequent plant regeneration of cryopreserved embryogenic calli of Hevea brasiliensis (Müll. Arg.). Plant Cell Rep 26:559–569
Le Roux Y, Pagès L (1994) Développement et polymorphisme racinaires chez de jeunes semis d’hévéa (Hevea brasiliensis). Can J Bot 72:924–932
Le Roux Y, Pagès L (1996) Réaction géotropique des différents types de racines chez l’hévéa (Hevea brasiliensis). Can J Bot 74:1910–1918
Liu WK, Yang QC (2008) Integration of mycorrhization and photoautotrophic micropropagation in vitro: feasibility analysis for mass production of mycorrhizal transplants and inoculants of arbuscular mycorrhizal fungi. Plant Cell Tiss Organ Cult 95:131–139
Louche-Tessandier D, Samson G, Hernández-Sebastià C, Chagvardieff P, Desjardins Y (1999) Importance of light and CO2 on the effects of endomycorrhizal colonization on growth and photosynthesis of potato plantlets (Solanum tuberosum) in an in vitro tripartite system. New Phytol 142:539–550
Martins A, Barroso J, Pais MS (1996) Effect of ectomycorrhizal fungi on survival and growth of micropropagated plants and seedlings of Castanea sativa mill. Mycorrhiza 6:265–270
McClelland MT, Smith MAL, Carothers ZB (1990) The effects of in vitro and ex vitro root initiation on subsequent microcutting root quality in three woody plants. Plant Cell Tiss Organ Cult 23:115–123
McGonigle TP, Miller MH, Evans DG, Fairchild GL, Swanand JA (1990) A new method which gives an objective measure of colonization of roots by vesicular–arbuscular mycorrhizal fungi. New Phytol 115:495–501
Mosaleeyanon K, Cha-um S, Kirdmanee C (2004) Enhanced growth and photosynthesis of rain tree (Samanea saman Merr.) plantlets in vitro under a CO2-enriched condition with decreased sucrose concentrations in the medium. Sci Hortic 103:51–63
Newman EI (1966) A method of estimating the total length of root in a sample. J Appl Ecol 3:136–145
Nowak J (1998) Benefits of in vitro “biotization” of plant tissue cultures with microbial inoculants in vitro. Cell Dev Biol Plant 34:122–130
Padilla IMG, Carmona E, Westendorp N, Encina CL (2006) Micropropagation and effects of mycorrhiza and soil bacteria on acclimatization and development of lucumo (Pouteria lucuma R. and Pav.) var. La Molina in vitro. Cell Dev Biol Plant 42:193–196
Pawlowska TE, Douds DD Jr, Charvat I (1999) In vitro propagation and life cycle of the arbuscular mycorrhizal fungus Glomus etunicatum. Mycol Res 103:1549–1556
Pospísilová J, Synková H, Haisel D, Semorádová S (2007) Acclimation of plantlets to ex vitro conditions: effects of air humidity, irradiance, CO2 concentration and abscisic acid (a review). Acta Hortic 748:29–38
Schwob I, Ducher M, Coudret A (1999) Effects of climatic factors on native arbuscular mycorrhizae and Meloidogyne exigua in a Brazilian rubber tree (Hevea brasiliensis) plantation. Plant Pathol 48:19–25
Staddon PL, Fitter AH (1998) Does elevated atmospheric carbon dioxide affect arbuscular mycorrhizas? Trends Ecol Evol 13:455–458
Staddon PL, Graves JD, Fitter AH (1998) Effect of enhanced atmospheric CO2 on mycorrhizal colonization by Glomus mosseae in Plantago lanceolata and Trifolium repens. New Phytol 139:571–580
Inc SS (2001) Statistica® Release 6. StatSoft Inc, Tulsa
Tawaraya K, Takaya Y, Turjaman M, Tuah SJ, Limin SH, Tamai Y, Cha JY, Wagatsuma T, Osaki M (2003) Arbuscular mycorrhizal colonization of tree species grown in peat swamp forests of Central Kalimantan, Indonesia. For Ecol Manage 182:381–386
Treseder KK, Allen MF (2000) Mycorrhizal fungi have a potential role in soil carbon storage under elevated CO2 and nitrogen deposition. New Phytol 147:189–200
Vaysse L, Bonfils F, Thaler P, Sainte-Beuve J (2009) Chapter 9.5. Natural rubber. In: Höffer R (ed) Sustainable solutions for modern economies. The Royal Society of Chemistry, Cambridge, pp 339–367
Vierheilig H, Coughlan AP, Wyss U, Piché Y (1998) Ink and vinegar, a simple staining technique for arbuscular mycorrhizal fungi. Appl Environ Microbiol 64:5004–5007
Voets L, de la Providencia IE, Fernandez K, IJdo M, Cranenbrouck S, Declerck S (2009) Extraradical mycelium network of arbuscular mycorrhizal fungi allows fast colonization of seedlings under in vitro conditions. Mycorrhiza 19:346–356
Acknowledgments
We are grateful to Dr. Pascal Montoro of the UMR Agap, Développement et Amélioration des Plantes, CIRAD-CP (France) for his technical advices. Sosa Rodríguez T. acknowledges the support of the “coopération au développement” Ph.D. Scholarship of the Université catholique de Louvain during this work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Editor: J. Forster
Rights and permissions
About this article
Cite this article
Sosa-Rodriguez, T., Dupré de Boulois, H., Granet, F. et al. In vitro mycorrhization of the rubber tree Hevea brasiliensis Müll Arg. In Vitro Cell.Dev.Biol.-Plant 49, 207–215 (2013). https://doi.org/10.1007/s11627-012-9485-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11627-012-9485-5