Summary
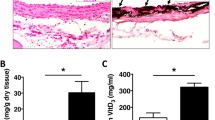
We have used in vivo balloon catheterization in combination with in vitro organ culture to develop a model system for vascular neointima formation. A Fogarty balloon catheter was used to deendothelialize and rupture the internal elastic lamina of aortae in adult rabbits. After three d of recovery, aortae were harvested, divided into segments, and placed into organ culture. We obtained a daily index of cell proliferation in cultured vessels using [3H]thymidine incorporation into DNA. Also, segments were collected and processed for routine histology or immunohistochemistry. Aortic segments that had undergone ballooning 3 d before harvest and then cultured exhibited diffuse neointimal growth after several d in vitro, whereas those from sham-operated (nonballooned) rabbits showed generally only a single endothelial cell layer that is characteristic of normal intima. Aortae that were harvested, balloon-damaged in vitro, and then cultured exhibited no neointimal growth. The neointima that developed in cultured segments from in vivo ballooned rabbits was primarily of smooth muscle cell origin as determined by positive immunostaining for α-smooth muscle actin. The intima:media thickness ratios were significantly higher in aortic segments from ballooned rabbits at harvest and after 4 or 7 d in culture compared with those from nonballooned rabbits. Also, the [3H]thymidine index was higher in the in vivo ballooned aorta compared to non-ballooned or in vitro ballooned vessel. We conclude that ballooning in vivo followed by exposure to blood-borne elements produces an enhanced proliferative response in cultured vessels that is distinct from other in vitro models of neointimal growth.
Similar content being viewed by others
References
Ausubel, F. M.; Brent, R.; Kingston, R. E., et al. Short protocols in molecular biology. A compendium of methods from current protocols in molecular biology. 2nd ed. New York: John Wiley & Sons; 1992.
Badimon, L.; Alfon, J.; Royo, T., et al. Cell biology of restenosis post-angioplasty. Z. Kardiol. 84 (suppl 4):145–149; 1995.
Boonen, H. C. M.; Schiffers, P. M. H.; Fazzi, G. E., et al. DNA synthesis in isolated arteries. Kinetics and structural consequences. Am. J. Physiol. 260:H210-H217; 1991.
Buck, R. C. Organ cultures of rat aorta: a scanning and transmission electron microscopic study. Exp. Mol. Pathol. 77:260–276; 1977.
Christensen, B. C.; Garbarsch, C. Repair in arterial tissue. A scanning electron microscopic (SEM) and light microscopic study on the endothelium of rabbit thoracic aorta following a single dilatation injury. Virchows Arch. Abt. A Pathol. Anat. 73:93–106; 1973.
Clausell, N.; de Lima, V. C.; Molossi, S., et al. Expression of tumour necrosis factor alpha and accumulation of fibronectin in coronary artery restenotic lesions retrieved by atherectomy. Br. Heart J. 95:534–539; 1995.
Clowes, A. W.; Clowes, M. M.; Reidy, M. A. Kinetics of cellular proliferation after arterial injury. III. Endothelial and smooth muscle growth in chronically denuded vessels. Lab. Invest. 86:295–303; 1986.
Edwards, I. J.; Wagner, W. D.; Owens, R. T. Macrophage secretory products selectively stimulate dermatan sulfate proteoglycan production in cultured arterial smooth muscle cells. Am. J. Pathol. 136:609–621; 1990.
Ehrlich, H. P. Culture of aorta. Methods Cell Biol. 21A:117–134; 1980.
Fagin, J. A.; Cercek, B.; Forrester, J. S. Restenosis: cellular mechanisms. In: Schwartz, R. S., ed. Coronary restenosis. Boston: Blackwell Scientific Publications; 1993:238–248.
Farhy, R. D.; Carretero, O. A.; Ho, K.-L., et al. Role of kinins and nitric oxide in the effects of angiotensin converting enzyme inhibitors on neointima formation. Circ. Res. 72:1202–1210; 1993.
Ferguson, J. J., III. Conventional antithrombotic approaches. Am. Heart J. 95:651–657; 1995.
Fingerle, J.; Faulmuller, A.; Muller, G., et al. Pituitary factors in blood plasma are necessary for smooth muscle cell proliferation in response to injury in vivo. Arterioscler. Thromb. 92:1488–1495; 1992.
Forrester, J. S.; Fishbein, M.; Helfant, R., et al. A paradigm for restenosis based on cell biology: clues for the development of new preventive therapies. J. Am. Coll. Cardiol. 17:758–769; 1991.
Gabbiani, G.; Kocher, O.; Bloom, W. S., et al. Actin expression in smooth muscle cells of rat aortic intimal thickening, human atheromatous plaque, and cultured rat aortic media. J. Clin. Invest. 73:148–152; 1984.
Gallo, R.; Hayes, R.; Badimon, L., et al. The role of thrombosis in restenosis. In: Feuerstein, G. Z., ed. Coronary restenosis from genetics to therapeutics. New York: Marcel Dekker, Inc.; 1997:241–255.
Gotlieb, A. I.; Boden, P. Porcine aortic organ culture: a model to study the cellular response to vascular injury. In Vitro 84:535–542; 1984.
Groves, P. H.; Banning, A. P.; Penny, W. J., et al. Kinetics of smooth muscle cell proliferation and intimal thickening in a pig carotid model of balloon injury. Atherosclerosis 95:83–96; 1995.
Hanke, H.; Hassenstein, S.; Ulmer, A., et al. Accumulation of macrophages in the arterial vessel wall following experimental balloon angioplasty. Eur. Heart J. 15:691–698; 1994.
Hanke, H.; Strohschneider, T.; Oberhoff, M., et al. Time course of smooth muscle cell proliferation in the intima and media of arteries following experimental angioplasty. Circ. Res. 67:651–659; 1990.
Hsu, S. M.; Raine, L.; Fanger, H. Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J. Histochem. Cytochem. 81:577–580; 1981.
Humphrey, W. R.; Simmons, C. A.; Toombs, C. F., et al. Induction of neointimal hyperplasia by coronary angioplasty balloon overinflation: comparison of feeder pigs to Yucatan minipigs. Am. Heart J. 94:20–31; 1994.
Jackman, R. W.; Anderson, S. K.; Sheridan, J. D. The aortic intima in organ culture. Response to culture conditions and partial endothelial denudation. Am. J. Pathol. 133:241–251; 1988.
Koo, E. W.; Gotlieb, A. I. The use of organ cultures to study vessel wall pathobiology. Scanning Microsc. 92:827–834; discussion 835, 1992.
Lee, P. C.; Gibbons, G. H.; Dzau, V. J. Cellular and molecular mechanisms of coronary artery restenosis. Coron. Artery Dis. 93:254–259; 1993.
Lyle, E. M.; Fujita, T.; Conner, M. W., et al. Effect of inhibitors of factor Xa or platelet adhesion, heparin, and aspirin on platelet deposition in an atherosclerotic rabbit model of angioplasty injury. J. Pharmacol. Toxicol. Methods 95:53–61; 1995.
Merrilees, M. J.; Scott, L. Organ culture of rat carotid artery: maintenance of morphological characteristics and of pattern of matrix synthesis. In Vitro 82:900–910; 1982.
Morisaki, N.; Koyama, N.; Kawano, M., et al. Human macrophages modulate the phenotype of cultured rabbit aortic smooth muscle cells through secretion of platelet-derived growth factor. Eur. J. Clin. Invest. 22:461–468; 1992.
Neumann, F. J.; Ott, I.; Gawaz, M., et al. Neutrophil and platelet activation at balloon-injured coronary artery plaque in patients undergoing angioplasty. J. Am. Coll. Cardiol. 96:819–824; 1996.
Pederson, D. C.; Bowyer, D. E. Endothelial injury and healing in vitro. Studies using an organ culture system. Am. J. Pathol. 119:264–272; 1985.
Puchtler, H.; Meloan, S. N. Orcein, collastin and pseudo-elastica: a reinvestigation of Unna’s concepts. Histochemistry 79:119–130; 1979.
Reidy, M. A.; Bendeck, M. P. The development of arterial lesions: a process controlled by multiple factors. In: Feuerstein, G. Z., ed. Coronary restenosis from genetics to therapeutics. New York: Marcel Dekker, Inc.; 1997:55–67.
Ross, R. George Lyman Duff Memorial Lecture. Atherosclerosis: a problem of the biology of arterial wall cells and their interactions with blood components. Arteriosclerosis 1:293–311; 1981.
Sade, R. M.; Folkman, J.; Cotran, R. S. DNA synthesis in endothelium of aortic segments in vitro. Exp. Cell Res. 74:297–306; 1972.
Schwartz, R. S.; Holmes, D. R., Jr.; Topol, E. J. The restenosis paradigm revisited: an alternative proposal for cellular mechanisms. J. Am. Coll. Cardiol. 20:1284–1293; 1992.
Schwartz, S. M.; Reidy, M. A.; O’Brien, E. R. Assessment of factors important in atherosclerotic occlusion and restenosis. Thromb. Haemostasis 95:541–551; 1995.
Slomp, J.; Gittenberger-deGroot, A. C.; van Munsteren, J. C., et al. Nature and origin of the neointima in whole vessel wall organ culture of the human saphenous vein. Virchows Archiv. 96:59–67; 1996.
Stevens, A. The haematoxylins. In: Bancroft, J. D.; Stevens, A., ed. Theory and practice of histological techniques. New York: Churchill Livingstone; 1990:107–118.
St. Clair, R. W.; Lofland, H. B., Jr. Uptake and esterification of exogenous cholesterol by organ cultures of normal and atherosclerotic pigeon aorta. Proc. Soc. Exp. Biol. Med. 71:632–637; 1971.
Wilcox, J. N. Molecular biology: insight into the causes and prevention of restenosis after arterial intervention. Am. J. Cardiol. 72:88E-95E; 1993.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Dale, W.E., Batra, P.S. & Blaine, E.H. Enhanced neointimal growth in cultured rabbit aorta following in vivo balloon angioplasty. In Vitro Cell.Dev.Biol.-Animal 34, 805–812 (1998). https://doi.org/10.1007/s11626-998-0035-8
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s11626-998-0035-8