Abstract
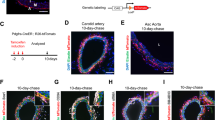
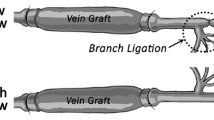
Accumulation of smooth muscle cells (SMC) results in neointima formation in injured vessels. Two graft models consisting of vein and artery grafts were created by anastomosing common carotid arteries to donor vessels. To identify the origin of the neointima cells from anastomosed arteries, we use Wnt1-Cre/reporter mice to label and track SMCs in the common carotid artery. The contribution of SMCs in the neighboring arteries to neointima formation was studied. On evaluating the artery grafts after 1 month, >90 % of the labeled neointima cells were found to have originated from the anastomosing host arteries. Most of the neointima cells were also smooth muscle α-actin positive (SMA-α+) and expressed the smooth muscle myosin heavy chain (SMMHC), the SMC terminal differentiation marker. In vein grafts, about 60 % SMA-α-positive cells were from anastomosing arteries. Bone marrow cells did not contribute to neointima SMCs in vein grafts, but did co-stain with markers of inflammatory cells. Wnt1 expression was not detected in the neointima cells in the vein or artery grafts, or the injured femoral arteries. Neointima SMCs showed the synthetic phenotype and were positively labeled with BrdU in vitro and in vivo. Treatment with the IGF-1 receptor inhibitor suppressed SMC proliferation and neointima formation in vein grafts. Our results indicate that SMCs from the neighboring artery are predominantly present in the neointima formed in both vein and artery grafts and that Wnt1-Cre mice can be used to explore the role of SMCs originating from neighboring vessels in vascular remodeling.







Similar content being viewed by others
Abbreviations
- VSMC:
-
Vascular smooth muscle cells
- NC:
-
Neural crest
- Wnt1:
-
Wingless-type MMTV integration site 1 family
References
Arciniegas E, Frid MG, Douglas IS, Stenmark KR (2007) Perspectives on endothelial-to-mesenchymal transition: potential contribution to vascular remodeling in chronic pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 293:L1–L8. doi:10.1152/ajplung.00378.2006
Bentzon JF, Weile C, Sondergaard CS, Hindkjaer J, Kassem M, Falk E (2006) Smooth muscle cells in atherosclerosis originate from the local vessel wall and not circulating progenitor cells in ApoE knockout mice. Arterioscler Thromb Vasc Biol 26:2696–2702. doi:10.1161/01.ATV.0000247243.48542.9d
Caplice NM, Wang S, Tracz M, Croatt AJ, Grande JP, Katusic ZS, Nath KA (2007) Neoangiogenesis and the presence of progenitor cells in the venous limb of an arteriovenous fistula in the rat. Am J Physiol Ren Physiol 293:F470–F475. doi:10.1152/ajprenal.00067.2007
Chen PY, Qin L, Barnes C, Charisse K, Yi T, Zhang X, Ali R, Medina PP, Yu J, Slack FJ, Anderson DG, Kotelianski V, Wang F, Tellides G, Simons M (2012) FGF regulates TGF-beta signaling and endothelial-to-mesenchymal transition via control of let-7 miRNA expression. Cell Rep 2:1684–1696. doi:10.1016/j.celrep.2012.10.021
Cheng J, Du J (2007) Mechanical stretch simulates proliferation of venous smooth muscle cells through activation of the insulin-like growth factor-1 receptor. Arterioscler Thromb Vasc Biol 27:1744–1751. doi:10.1161/ATVBAHA.107.147371
Cheng J, Wang Y, Liang A, Jia L, Du J (2012) FSP-1 silencing in bone marrow cells suppresses neointima formation in Vein Graft. Circ Res 110:230–240. doi:10.1161/circresaha.111.246025
Cooley BC (2004) Murine model of neointimal formation and stenosis in vein grafts. Arterioscler Thromb Vasc Biol 24:1180–1185. doi:10.1161/01.ATV.0000129330.19057.9f
Diez M, Musri MM, Ferrer E, Barbera JA, Peinado VI (2010) Endothelial progenitor cells undergo an endothelial-to-mesenchymal transition-like process mediated by TGFbetaRI. Cardiovasc Res 88:502–511. doi:10.1093/cvr/cvq236
Echelard Y, Vassileva G, McMahon AP (1994) Cis-acting regulatory sequences governing Wnt-1 expression in the developing mouse CNS. Development 120:2213–2224
Engelse MA, Lardenoye JHP, Neele JM, Grimbergen JM, de Vries MR, Lamfers MLM, Pannekoek H, Quax PHA, de Vries CJM (2002) Adenoviral activin a expression prevents intimal hyperplasia in human and murine blood vessels by maintaining the contractile smooth muscle cell phenotype. Circ Res 90:1128–1134. doi:10.1161/01.res.0000021044.53156.f5
Goldman S, Zadina K, Moritz T, Ovitt T, Sethi G, Copeland JG, Thottapurathu L, Krasnicka B, Ellis N, Anderson RJ, Henderson W (2004) Long-term patency of saphenous vein and left internal mammary artery grafts after coronary artery bypass surgery: results from a Department of Veterans Affairs Cooperative Study. J Am Coll Cardiol 44:2149–2156. doi:10.1016/j.jacc.2004.08.064
Hagensen MK, Shim J, Falk E, Bentzon JF (2011) Flanking recipient vasculature, not circulating progenitor cells, contributes to endothelium and smooth muscle in murine allograft vasculopathy. Arterioscler Thromb Vasc Biol 31:808–813. doi:10.1161/atvbaha.110.221184
Hoglund VJ, Dong XR, Majesky MW (2010) Neointima Formation. Arterioscler Thromb Vasc Biol 30:1877–1879. doi:10.1161/atvbaha.110.211433
Hoofnagle MH, Thomas JA, Wamhoff BR, Owens GK (2006) Origin of neointimal smooth muscle: we’ve come full circle. Arterioscler Thromb Vasc Biol 26:2579–2581. doi:10.1161/01.ATV.0000249623.79871.bc
Hu Y, Bock G, Wick G, Xu Q (1998) Activation of PDGF receptor alpha in vascular smooth muscle cells by mechanical stress. Faseb J 12:1135–1142
Hu Y, Davison F, Ludewig B, Erdel M, Mayr M, Url M, Dietrich H, Xu Q (2002) Smooth muscle cells in transplant atherosclerotic lesions are originated from recipients, but not bone marrow progenitor cells. Circulation 106:1834–1839. doi:10.1161/01.cir.0000031333.86845.dd
Hu Y, Xu Q (2002) New Mouse Model of Vein Bypass Graft Atherosclerosis. Heart Lung Circ 11:182–188. doi:10.1046/j.1444-2892.2002.00138.x
Hu Y, Zhang Z, Torsney E, Afzal AR, Davison F, Metzler B, Xu Q (2004) Abundant progenitor cells in the adventitia contribute to atherosclerosis of vein grafts in ApoE-deficient mice. J Clin Investig 113:1258–1265. doi:10.1172/JCI19628
Huang J, Cheng L, Li J, Chen M, Zhou D, Lu MM, Proweller A, Epstein JA, Parmacek MS (2008) Myocardin regulates expression of contractile genes in smooth muscle cells and is required for closure of the ductus arteriosus in mice. J Clin Invest 118:515–525. doi:10.1172/JCI33304
Hutson MR, Kirby ML (2007) Model systems for the study of heart development and disease. Cardiac neural crest and conotruncal malformations. Semin Cell Dev Biol 18:101–110. doi:10.1016/j.semcdb.2006.12.004
Iwano M, Neilson EG (2004) Mechanisms of tubulointerstitial fibrosis. Curr Opin Nephrol Hypertens 13:279–284. doi:10.1097/01.mnh.0000126791.93346.4a
Kennedy E, Hakimjavadi R, Greene C, Mooney CJ, Fitzpatrick E, Collins LE, Loscher CE, Guha S, Morrow D, Redmond EM, Cahill PA (2014) Embryonic rat vascular smooth muscle cells revisited—a model for neonatal, neointimal SMC or differentiated vascular stem cells? Vascular Cell 6:6. doi:10.1186/2045-824X-6-6
Kobayashi K, Yokote K, Fujimoto M, Yamashita K, Sakamoto A, Kitahara M, Kawamura H, Maezawa Y, Asaumi S, Tokuhisa T, Mori S, Saito Y (2005) Targeted disruption of TGF-{beta}-Smad3 signaling leads to enhanced neointimal hyperplasia with diminished matrix deposition in response to vascular injury. Circ Res 96:904–912. doi:10.1161/01.res.0000163980.55495.44
Mead TJ, Yutzey KE (2012) Notch pathway regulation of neural crest cell development in vivo. Dev Dyn 241:376–389. doi:10.1002/dvdy.23717
Motwani JG, Topol EJ (1998) Aortocoronary saphenous vein graft disease: pathogenesis, predisposition, and prevention. Circulation 97:916–931. doi:10.1161/01.CIR.97.9.916
Muzumdar MD, Tasic B, Miyamichi K, Li L, Luo L (2007) A global double-fluorescent Cre reporter mouse. Genesis 45:593–605. doi:10.1002/dvg.20335
Scott NA, Cipolla GD, Ross CE, Dunn B, Martin FH, Simonet L, Wilcox JN (1996) Identification of a potential role for the adventitia in vascular lesion formation after balloon overstretch injury of porcine coronary arteries. Circulation 93:2178–2187. doi:10.1161/01.CIR.93.12.2178
Shi Y, O’Brien JE, Fard A, Mannion JD, Wang D, Zalewski A (1996) Adventitial myofibroblasts contribute to neointimal formation in injured porcine coronary arteries. Circulation 94:1655–1664. doi:10.1161/01.CIR.94.7.1655
Shi Y, O’Brien JE Jr, Mannion JD, Morrison RC, Chung W, Fard A, Zalewski A (1997) Remodeling of autologous saphenous vein grafts. The role of perivascular myofibroblasts. Circulation 95:2684–2693. doi:10.1161/01.CIR.95.12.2684
Shi Y, Pieniek M, Fard A, O’Brien J, Mannion JD, Zalewski A (1996) Adventitial remodeling after coronary arterial injury. Circulation 93:340–348. doi:10.1161/01.CIR.93.2.340
Shimizu T, De Wispelaere A, Winkler M, D’Souza T, Caylor J, Chen L, Dastvan F, Deou J, Cho A, Larena-Avellaneda A, Reidy M, Daum G (2012) Sphingosine-1-Phosphate Receptor 3 Promotes Neointimal Hyperplasia in Mouse Iliac-Femoral Arteries. Arterioscler Thromb Vasc Biol 32:955–961. doi:10.1161/atvbaha.111.241034
Singh N, Trivedi CM, Lu M, Mullican SE, Lazar MA, Epstein JA (2011) Histone Deacetylase 3 Regulates Smooth Muscle Differentiation in Neural Crest Cells and Development of the Cardiac Outflow Tract/Novelty and Significance. Circ Res 109:1240–1249. doi:10.1161/circresaha.111.255067
Strieter RM, Keeley EC, Hughes MA, Burdick MD, Mehrad B (2009) The role of circulating mesenchymal progenitor cells (fibrocytes) in the pathogenesis of pulmonary fibrosis. J Leukoc Biol 86:1111–1118. doi:10.1189/jlb.0309132
Tanaka K, Sata M, Natori T, Kim-Kaneyama JR, Nose K, Shibanuma M, Hirata Y, Nagai R (2008) Circulating progenitor cells contribute to neointimal formation in nonirradiated chimeric mice. Faseb J 22:428–436
Tang Z, Wang A, Yuan F, Yan Z, Liu B, Chu JS, Helms JA, Li S (2012) Differentiation of multipotent vascular stem cells contributes to vascular diseases. Nat Commun 3:875. doi:10.1038/ncomms1867
Waldo KL, Kumiski D, Kirby ML (1996) Cardiac neural crest is essential for the persistence rather than the formation of an arch artery. Dev Dyn 205:281–292. doi:10.1002/(SICI)1097-0177(199603
Werner N, Nickenig G (2006) Influence of cardiovascular risk factors on endothelial progenitor cells: limitations for therapy? Arterioscler Thromb Vasc Biol 26:257–266. doi:10.1161/01.ATV.0000198239.41189.5d
Zernecke A, Schober A, Bot I, von Hundelshausen P, Liehn EA, Mopps B, Mericskay M, Gierschik P, Biessen EA, Weber C (2005) SDF-1α/CXCR4 axis is instrumental in neointimal hyperplasia and recruitment of smooth muscle progenitor cells. Circ Res 96:784–791. doi:10.1161/01.res.0000162100.52009.38
Zhang L, Freedman NJ, Brian L, Peppel K (2004) Graft-Extrinsic Cells Predominate in Vein Graft Arterialization. Athro Throm Vascul Biol 24:470–476. doi:10.1161/01.atv.0000116865.98067.31
Zou Y, Dietrich H, Hu Y, Metzler B, Wick G, Xu Q (1998) Mouse Model of Venous Bypass Graft Arteriosclerosis. Am J Pathol 153:1301–1310. doi:10.1152/ajplung.00378.2006
Acknowledgments
We acknowledge Dr. William E Mitch for his constructive suggestions. We also thank Dr. Abha Sharma for critically reading and modifying the manuscript. This work was supported by grants from RO1 DK095867, the American Heart Association Grant 10SDG2780009 (to J.C.), the National Institutes of Health grants R37 and DK37175, and a generous grant from Dr. and Mrs. Harold Selzman.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liang, M., Liang, A., Wang, Y. et al. Smooth muscle cells from the anastomosed artery are the major precursors for neointima formation in both artery and vein grafts. Basic Res Cardiol 109, 431 (2014). https://doi.org/10.1007/s00395-014-0431-z
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00395-014-0431-z