Abstract
There has been much interest in the use of cell culture models of neurones, to avoid the animal welfare and cost issues of using primary and human-induced pluripotent stem cell (hiPSC)-derived neurones respectively. The human neuroblastoma cell line, SH-SY5Y, is extensively used in laboratories as they can be readily expanded, are of low cost and can be differentiated into neuronal-like cells. However, much debate remains as to their phenotype once differentiated, and their ability to recapitulate the physiology of bona fide neurones. Here, we characterise a differentiation protocol using retinoic acid and BDNF, which results in extensive neurite outgrowth/branching within 10 days, and expression of key neuronal and synaptic markers. We propose that these differentiated SH-SY5Y cells may be a useful substitute for primary or hiPSC-derived neurones for cell biology studies, in order to reduce costs and animal usage. We further propose that this characterised differentiation timecourse could be used as an in vitro model for neuronal differentiation, for proof-of principle studies on neurogenesis, e.g. relating to neurodegenerative diseases. Finally, we demonstrate profound changes in Tau phosphorylation during differentiation of these cells, suggesting that they should not be used for neurodegeneration studies in their undifferentiated state.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Reliable cell culture models of neurones are notoriously problematic. As a general rule, researchers either use primary neurones isolated from rodents (e.g. Sahu et al. 2019) or, more recently, human-induced pluripotent stem cell-derived (hIPSC) neurones e.g. (Stathakos et al. 2021). Both of these systems have their own merits and disadvantages, however both are extremely costly and therefore off limits to all but the most highly funded laboratories in high-income countries. In addition, these models are labour intensive and not applicable to high-throughput applications. There is therefore considerable interest in a cost-effective, reproducible cell model which can be readily scaled up to increase the capacity for repetition and reproducibility. Additionally, whilst it is certainly true that 2D cell monocultures fail to accurately represent the complex cellular interactions seen in all multicellular organisms, conversely they provide a simplified, precisely defined model in which to study the individual signalling pathways, the dysfunction of which is the basis of most diseases.
Many different studies have utilised the human-derived neuroblastoma cell line, SH-SY5Y as an in vitro model of neurones, e.g. ( Xicoy et al. 2017; Peng et al. 2021; Bell and Zempel 2022). This cell line is originally derived from the SK-N-SH cell line taken from a metastatic bone biopsy from a 4 year old patient. Under basal tissue culture conditions, these cells can be rapidly expanded and display an epithelial-like morphology. However, several different protocols exist to differentiate these cells into a more neuronal phenotype. These protocols generally involve serum restriction and exposure to retinoic acid (RA) (Cheung et al. 2009), with some protocols utilising nerve growth factor (NGF) (Shipley et al. 2016) and/or brain-derived neurotrophic factor (BDNF) (De Medeiros et al. 2019; Encinas et al. 2000). Differentiation of these cells results in extensive neurite outgrowth and branching, coupled with the expression of markers of mature neurones (e.g. NeuN, β-III tubulin) (Hromadkova et al. 2020), with different studies reporting that the resulting cells display either a dopaminergic (Niaz et al. 2021) or cholinergic (De Medeiros et al. 2019) phenotype. This is of great interest to researchers in the field of neurodegeneration, as these dopaminergic and cholinergic neurones display selective vulnerability in Parkinson’s Disease (Poewe et al. 2017) and Alzheimer’s Disease (Masters et al. 2015) respectively. However, few studies to date have investigated the ability of these differentiated cells to form synapses or characterised the timecourse of their differentiation. Such characterisation is potentially extremely useful due to the observation that neurogenesis in the dentate gyrus is impaired in both animal models and human Alzheimer’s disease (Moreno-Jiménez et al. 2019; Zhou et al. 2023), as it would allow the use of SH-SY5Y cells as an in vitro model for neuronal differentiation.
In this study, we have used a differentiation protocol based on the work of de Medieros (de Medieros et al., 2019), which uses both RA and BDNF. We confirm their findings that this protocol results in a robust phenotype of differentiated, neuronal-like cells, with extensive neurite outgrowth and branching. Further, we show that these cells express key pre- and post-synaptic markers, and markers of cholinergic and glutamatergic, but not dopaminergic neurones. Further, we demonstrate consistent changes in Tau phosphorylation during differentiation. Taken together, these data imply that this differentiation protocol might be a useful cell-line model for studying disruptions of neuronal differentiation/neurogenesis observed in the AD brain.
Materials and methods
SH-SY5Y Cell Culture
The human neuroblastoma SH-SY5Y cell line was obtained from American Type Culture Collection (ATCC, Manassas, VA). Cells were cultured in Dulbecco’s modified Eagle’s medium F-12 (DMEM/F-12; Gibco, Grand Island, NY, 11,524,436) with GlutaMAX™ supplement, 10% fetal bovine serum (FBS; Gibco, 11,514,436) and 1% non-essential amino acids (NEAAs; Gibco, 11,140,050). Cells were maintained at 37 °C and 5% CO2 in humidified air. Once wells reached 70–80% confluency, the cells were subcultured and used up to passage 20. Cells were regularly checked for mycoplasma contamination (Eurofins), returning negative results on every occasion.
Differentiation time course
On Day -1, cells were seeded at a density of 10,526 cells/cm2 in DMEM/F-12 supplemented with 10% FBS and 1% NEAAs. On Day 0, the cells were washed with 1 × phosphate buffer saline (PBS) and medium was replaced with diff1 media (DMEM/F-12, 2% FBS, 1% NEAAs, 10 μM RA). On Day 3 and 6, cells were washed, and the medium was replaced with diff2 media (DMEM/F-12, 1% FBS, 1% NEAAs, 10 μM RA, 50 ng/ml BDNF). See Fig. 1.
Diagrammatic representation of the differentiation protocol. SH-SY5Y cells are seeded at 40,000 cells per well of a 12-well plate in complete media at D-1. This media is changed to diff1 media at D0 (10 uM RA, 2% FBS), followed by diff2 media at D3 (10 uM RA, 50 ng/ml BDNF, 1% FBS). A further media change into fresh diff2 media is performed at D6. Cells are lysed for timepoints at D0, D3, D7 and D10.
Cell Lysis + Western blotting
Cells in 12-well plates were lysed on Day 0, 3, 7 and 10 in ice-cold lysis buffer (25 mM Hepes, 150 mM NaCl, 0.1% SDS, 1% triton, phosphatase/protease inhibitor tablet, pH 7.4). Cells were spun by centrifugation (20,000 xg, 4 °C, 5 min) and supernatant stored at -20 °C until required. Protein concentrations were quantified using a Bradford assay and diluted with lysis buffer to equal concentrations. Protein samples were heated at 75 °C for 5 min, separated by 12% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to methanol-activated polyvinylidene (PVDF) membrane. Membranes were blocked in 5% milk in 1 × tris-buffered saline-tween 20 (TBST; 10 mM Tris–HCl (pH 7.6), 150 mM NaCl, and 0.1% Tween-20) for 1 h at room temperature (RT) and incubated with primary antibodies (Table 1) in 5% milk at 4 °C overnight. Membranes were subsequently washed and incubated with secondary antibodies (Table 2) in 5% milk for 1–2 h in the dark at RT. Membranes were treated with HRP substrate and analysed using LICOR Image Studio software. All protein levels were normalised to GAPDH levels.
Immunocytochemistry
Cells were seeded on Geltrex-coated coverslips in 24-well plates. 10 × Geltrex was thawed with ice-cold serum free (SF) DMEM/F12 media and diluted to 0.25 × Geltrex with SF DMEM/F-12. 80 µL of 0.25 × Geltrex was added to each coverslip (1.3 cm2) and incubated for at least 20 min at 37 °C. Geltrex was removed, and cells (10,526 cells/cm2) were added to each coverslip and incubated for 4 h before each well was flooded with 200 µL DMEM/F-12 (10% FBS, 1% NEAAs).
After the differentiation protocol was followed, cells were fixed with 4% paraformaldehyde for 15 min at RT. Cells were washed with 1 × PBS three times and stored overnight at 4 °C. Blocking/permeabilisation was carried out in 10% horse serum, 1% BSA, in 0.1% PBTx (1 × PBS + 0.1% Triton-X) for 1 h. Primary antibodies β-III tubulin, PSD-95 and synaptophysin (Table 1) were incubated in 1% horse serum, 0.1% BSA in 0.1% PBTx overnight at 4 °C in a humid chamber with 1 × PBS. Coverslips were washed three times with 1 × PBS and incubated with secondary fluorescent antibodies (Table 2; Alexa Fluor 488 (AF488) and AF568 at a 1:1000 dilution) for 1–2 h at RT. Cells were washed and cell nuclei were co-stained using a DAPI solution for 5 min. Cells were washed again, and coverslips were mounted onto slides in MOWIOL (25 mg/ml DABICO) media. Cells were visualised and the fluorescent intensities were analysed using a confocal microscope and ImageJ software.
Statistical analysis
Quantitative data are expressed as mean ± standard error of the mean (SEM) from three independent experiments. Statistical analyses were performed using Prism 9.5.1 and p-value was determined as ns, * p > 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001. Precise statistical tests used will be indicated in individual results.
Results and discussion
Several papers document a variety of differentiation protocols for SH-SY5Y cells, and these protocols have been characterised to differing extents. As our work is principally focussed on neurodegenerative diseases, we were interested in protocols reporting to induce cholinergic or dopaminergic neuronal phenotypes, as these are relevant to Alzheimer’s Disease and Parkinson’s Disease, respectively. For this reason, we focussed on a differentiation protocol using both retinoic acid (RA) and brain-derived neurotrophic factor (BDNF), with a 10-day timecourse (De Medeiros et al. 2019) (Fig. 1). Importantly, this protocol is easy to perform without complex or expensive media preparations; requires no extra cell passaging (in comparison to some published protocols (Shipley et al. 2016)); and results in lysates with sufficient protein concentration for Western blotting-based quantification of major neuronal proteins (see Figs. 3, 5 and 6).
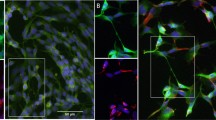
Using this protocol, we observed profound and robust morphological changes over the 10-day timecourse. During this time, the SH-SY5Y cells went from a largely epithelial morphology (Fig. 2A) to an increasingly neurone-like morphology with extensive neurite outgrowth and branching by Day 10 (Fig. 2B,C and D). Additionally, these neurites stained strongly positive for the neurone-specific β-III tubulin (Fig. 2E–H). Therefore, the differentiation protocol we have employed results in neuronal morphology in 10 d, driven by β-III tubulin polymerisation along neurites.
The differentiation protocol leads to reliable and robust outgrowth of neurites expressing β-III tubulin within 10 d. Transmitted light (left) and immunocytochemistry (ICC, right) images of SH-SY5Y cells at different timepoints during differentiation ((A): Day 0, (B): Day 3, (C): Day 7 and (D): Day 10) showing morphological changes during the differentiation. ICC images are stained for β-III tubulin (green) and DAPI (blue). Images are the composite of 33 confocal slices. Note that the transmitted light and ICC images are taken from different batches of cells. Scale bar = 50 µM.
To further characterize this differentiation protocol, we investigated and quantified the expression of key neuronal and synaptic markers during this timecourse using Western blotting (Fig. 3A-C). As expected from the ICC results in Fig. 2, we observed a significant and robust induction of β-III tubulin expression across the timecourse of differentiation, reflecting neurite formation. Additionally, we observed a strong induction of the expression of both the pre-synaptic marker, synaptophysin (Fig. 3B) and the post-synaptic marker, PSD-95 (Fig. 3C). Whilst both markers showed a significant increase over the timecourse of differentiation, it is interesting to note that PSD-95 expression increased most strongly between days 0–3, whereas synaptophysin expression increased most strongly between days 3–7. These results therefore indicate that, as well as neuronal morphology, differentiated SH-SY5Y cells may be forming synaptic structures.
Time-dependent expression of key neuronal and synaptic markers during differentiation of SH-SY5Y cells. All experiments involved SDS-PAGE and Western blotting of whole cell lysates at the designated timepoints. (A): Expression of β-III tubulin. Top: quantification of the relative expression levels normalised to D10. Bottom: representative Western blot. (B): Expression of synaptophysin. Top: quantification of the relative expression levels normalised to D10. Bottom: representative Western blot. (C): Expression of PSD-95. Top: quantification of the relative expression levels normalised to D10. Bottom: representative Western blot. For all graphs, values are mean ± SEM (n = 3). * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001. 1-way ANOVA with Tukey’s post-hoc test.
Having established that this differentiation protocol results in the expression of synaptic markers, we next used immunocytochemistry to characterise the localisation of these proteins. Our reasoning was that if SH-SY5Y cells differentiated using this protocol are capable of synapse formation, we would observe punctate distribution of both the post-synaptic marker PSD-95 and the pre-synaptic marker synaptophysin. As shown in Fig. 4A-C, synaptophysin showed a neurite-localised, punctate distribution by Day 10. This is what we would expect if presynaptic structures were being formed in these cells, and is broadly similar to synaptophysin staining observed in primary neurones, e.g. (Swarnkar et al. 2021). We observed a similar distribution of PSD-95 along neurite processes (Fig. 4D-F), which was possibly even more punctate than that of synaptophysin in places. Whilst it is notable that both synaptic markers are visible throughout the cell bodies at Day 10, these results nevertheless suggest that this differentiation protocol leads to the formation of both pre- and post-synaptic structures. We note, however, that we should be cautious in over-interpreting these data, and that the puncta we show here may simply represent transport cargo. However, costaining for both PSD-95 and synaptophysin (Fig. 4G-I) revealed several sites where these pre- and post-synaptic markers were in close apposition. Whilst we acknowledge that this does not provide definitive evidence, it is however consistent with the formation of synaptic structures.
Punctate distribution of pre- and post-synaptic markers along neurites of D10 differentiated SH-SY5Y cells. (A-C): Immunocytochemistry for DAPI (blue, A), synaptophysin (green, B) and merged (C) in SH-SH5Y cells after 10 d of differentiation, showing diffuse somatic and more punctate neurite localisation. Images are the composite of 33 confocal slices. (D-F): Immunocytochemistry for DAPI (blue, D), PSD-95 (green, E) and merged (F) in SH-SH5Y cells after 10 d of differentiation, showing diffuse somatic and more punctate neurite localisation. (G-I): Immunocytochemistry for PSD-95 (green, G), synaptophysin (red, H) and merged (I). Arrows in I indicate areas of close apposition of PSD-95 and synaptophysin staining. Images are the composite of 32 confocal slices. Scale bar = 50 µM.
There are differing reports as to the neuronal subtype best represented by differentiated SH-SY5Y cells, with different protocols reporting either a dopaminergic (Niaz et al. 2021) or cholinergic phenotype (De Medeiros et al. 2019). For this reason, we used Western blotting to examine the presence and relative levels of markers of different neuronal subtypes. Consistent with our differentiated SH-SY5Y cells having a cholinergic phenotype, we observed a robust increase in the expression of choline acetyltransferase (ChAT) over the timecourse of differentiation (Fig. 5A). In contrast, expression of tyrosine hydroxylase (TH), a key enzyme in dopamine synthesis, was expressed at high levels on Day 0, however this level rapidly decreased as the cells differentiated, becoming almost undetectable by Day 10 (Fig. 5B). Interestingly, expression of glutamate-aspartate transporter (GLAST), a key protein in cellular glutamate uptake and often used as a marker of glutamatergic neurones (Rodríguez-Campuzano and Ortega 2021), was also increased during differentiation (Fig. 5C), implying that the fully differentiated cells have either a mixed cholinergic and glutamatergic phenotype or that there is a mixed population. Interestingly, glutamine synthetase (GLUL) levels did not change significantly during our differentiation protocol (Fig. 5D), implying that either this is not a reliable marker of glutamatergic neurones, or that our protocol results in an incomplete glutamatergic phenotype. It should be noted that there is scant evidence in the literature of differentiated SH-SY5Y cells expressing AMPA or NMDA receptors, although some studies have demonstrated the expression of mRNA (Anchesi et al. 2023) and protein (Yang et al. 2020) of both under certain conditions (often a combination of RA and other factors, e.g. GLP-1 or THC).
Differentiated cells display a mixed cholinergic/glutamatergic phenotype. All experiments involved SDS-PAGE and Western blotting of whole cell lysates at the designated timepoints. (A): Expression of choline acetyltransferase (ChAT). Top: quantification of the relative expression levels normalised to D10. Bottom: representative Western blot. (B): Expression of tyrosine hydroxylase (TH). Top: quantification of the relative expression levels normalised to D10. Bottom: representative Western blot. (C): Expression of glutamate-aspartate transporter (GLAST). Top: quantification of the relative expression levels normalised to D10. Bottom: representative Western blot. (D): Expression of glutamine synthetase (GLUL). Top: quantification of the relative expression levels normalised to D10. Bottom: representative Western blot. No significant differences observed. For all graphs, values are mean ± SEM (n = 3). 1-way ANOVA with Tukey’s post-hoc test. * p < 0.05, ** p < 0.01, *** p < 0.001, ****, p < 0.0001.
Tau is a microtubule-binding protein which plays important roles in neuronal growth and development including microtubule polymerisation and stabilisation e.g. (Kadavath et al. 2015), however, it is best known as the major component of neurofibrillary tangles (NFTs) in Alzheimer’s disease (Masters et al. 2015). Indeed several studies have indicated that cognitive decline correlates more closely with the extent of Tau pathology (termed Braak staging) than any other pathological hallmark of Alzheimer’s Disease (Lowe et al. 2018). In NFTs, Tau is typically found in a hyperphosphorylated state, leading to the general view that all Tau phosphorylation is neurotoxic. However, this is not always the case, and Tau phosphorylation (even at residues associated with AD-pathology) has been demonstrated to occur during development of neurones and the nervous system (Fuster-Matanzo et al. 2009, 2012; Hefti et al. 2019). We therefore examined differentiation-dependent regulation of phosphorylation of Tau at three specific residues, S202/T205 (collectively known as the AT8 epitope) and pS396, which are all associated with AD pathology. The pS396 antibody detects bands of 4 different molecular weights – at ~ 50 kDa (presumably representing pS396 monomeric Tau), at > 100 kDa band (presumably oligomers or dimers of pS396 Tau) and two bands between, at approximately 55 and 70 kDa, which we presume represent either multiple phosphorylated forms of Tau or different isoforms. Interestingly, we noticed a reciprocal relationship between the 100 kDa band and the lower molecular weight bands during differentiation, with the 50, 55 and 70 kDa bands increasing and the 100 kDa band decreasing in abundance between Day 0 and Day 10 (Fig. 6A-D). These results suggest that during neuronal differentiation, whilst phosphorylation of Tau at pS396 increases, the formation of higher-order structures decreases. In contrast, for the S202/T205 epitope, we noted a consistent increase in Tau phosphorylation across the timecourse. In our hands, this antibody also revealed multiple bands between 75 kDa and > 200 kDa, however it was the doublet at around 78 kDa (the predicted molecular weight according to the manufacturer, see Table 1 for details) which showed a strong developmental trend (Fig. 6E and F). It should also be noted that total Tau expression is strongly induced by our differentiation protocol (Fig. 6G), indicating that this is potentially behind the increase in abundance of phosphorylated species of Tau seen in this study. Importantly, however this increase in total Tau expression is in contrast to the decrease in pS396 Tau oligomers. Whilst we cannot ascribe a specific function to these phosphorylated Tau species in differentiation, the fact that they are differentially developmentally regulated implies that dysregulation of neurogenesis, known to occur in AD, might be an instigator of aberrant Tau phosphorylation. Interestingly, both the S396 and S202/T205 epitopes have been reported to be phosphorylated by CDK5/p35 and GSK3β (Johnson and Stoothoff 2004; Li et al. 2006; Gao et al. 2020), implying that these kinases are activated during the process of differentiation in SH-SY5Y cells. Indeed, this observation is in line with studies demonstrating a critical role for CDK5 in adult hippocampal neurogenesis (Lagace et al. 2008). Thus, the SH-SY5Y differentiation protocol outlined in this study represents a viable and robust model to investigate this.
Tau phosphorylation at residues associated with AD pathology displays differentiation-dependent regulation. All experiments involved SDS-PAGE and Western blotting of whole cell lysates at the designated timepoints. (A-C): Quantification of 3 separate pS396 bands detected on Western blot. These bands are ~ 100 kDa ((A), presumably dimers of phosphorylated Tau (pTau)), ~ 75 kDa ((B), presumably either a multiply phosphorylated monomeric pTau or a monomer of a longer isoform also detected by this antibody) and ~ 50 kDa ((C), presumably monomeric pTau). Bands quantified are indicated in representative Western blot (D). Quantification of the relative signal is normalised to D10. Values are mean ± SEM (n = 3). Bottom: representative Western blot. (E): Quantification of S202/T205 phosphorylation epitope (also referred to as AT8), relative signal levels normalised to D10. (F): representative Western blot, with the 78 kDa band quantified indicated with an arrow. (G): Above: Total tau quantification, relative signals normalised to D10. Below: Representative Western blot of total tau and GAPDH. For all graphs, values are mean ± SEM (n = 3). 1-way ANOVA with Tukey’s post-hoc test. * p < 0.05, ** p < 0.01, *** p < 0.001, ****, p < 0.0001.
Conclusions
In this study, we have provided further evidence that the SH-SY5Y cell line can be reliably differentiated into mature neuronal-like cells, displaying a cholinergic/glutamatergic phenotype, using serum restriction, retinoic acid and BDNF. We have also demonstrated that these differentiated cells exhibit a punctate distribution of key synaptic markers along neurites. Many studies use these cells in their undifferentiated state, but our data argue that this is unwise due to the lack of expression of neuronal proteins. This is in agreement with a recent study showing changes in APP expression and localisation during differentiation in SH-SY5Y cells (Riegerová et al. 2021). These data support previous studies (De Medeiros et al. 2019; Hromadkova et al. 2020) and further suggest that this differentiation protocol is a suitable model for the study of neurogenesis and neuronal differentiation. It is now widely accepted that adult hippocampal neurogenesis occurs in humans, and moreover continues throughout life. Furthermore, this neurogenesis declines with age, and is particularly attenuated in Alzheimer’s disease (Moreno-Jiménez et al. 2019). It is noteworthy that one of the first sites affected in AD, the dentate gyrus of the hippocampus, is also one of the main sites of adult neurogenesis (Kempermann et al. 2018). Our demonstration that Tau phosphorylation is regulated during differentiation of SH-SY5Y cells implies that dysfunctional differentiation of neuronal precursors in AD may be a contributing factor in aberrant Tau phosphorylation and Tau pathology. We therefore propose that this SH-SY5Y differentiation protocol is a useful model to study this process. Future potential investigations could include studies on the effects of beta-amyloid on this differentiation process, the effect of neuroinflammatory factors, environmental toxins, e.g. ethanol or nanoplastics, dietary factors and the influence of ischaemia, all of which have been suggested to play a role on AD development. Additionally, this system is highly amenable to genetic manipulation to investigate the roles of the many different human polymorphisms associated with AD risk. Whilst we fully acknowledge that our 2D monoculture system does not recapitulate the exquisite complexity of the central nervous system, we nevertheless believe it will prove useful for proof-of-concept investigations or high throughput assays (due to its low cost), which can then be extended into more physiologically relevant systems.
Data availability
All raw data is available from the authors on request.
References
Anchesi I, Schepici G, Chiricosta L, Gugliandolo A, Salamone S, Caprioglio D, Pollastro F, Mazzon E (2023) Δ8-THC induces up-regulation of glutamatergic pathway genes in differentiated SH-SY5Y: a transcriptomic study. Int J Mol Sci 24:9486. https://doi.org/10.3390/ijms24119486
Bell M, Zempel H (2022) SH-SY5Y-derived neurons: a human neuronal model system for investigating TAU sorting and neuronal subtype-specific TAU vulnerability. Rev Neurosci 33:1–15. https://doi.org/10.1515/revneuro-2020-0152
Cheung Y-T, Lau WK-W, Yu M-S, Lai CS-W, Yeung S-C, So K-F, Chang RC-C (2009) Effects of all-trans-retinoic acid on human SH-SY5Y neuroblastoma as in vitro model in neurotoxicity research. Neurotoxicology 30:127–135. https://doi.org/10.1016/j.neuro.2008.11.001
De Medeiros LM, De Bastiani MA, Rico EP, Schonhofen P, Pfaffenseller B, Wollenhaupt-Aguiar B, Grun L, Barbé-Tuana F, Zimmer ER, Castro MAA, Parsons RB, Klamt F (2019) Cholinergic differentiation of human neuroblastoma SH-SY5Y cell line and its potential use as an in vitro model for Alzheimer’s disease studies. Mol Neurobiol 56:7355–7367. https://doi.org/10.1007/s12035-019-1605-3
Encinas M, Iglesias M, Liu Y, Wang H, Muhaisen A, Ceña V, Gallego C, Comella JX (2000) Sequential treatment of SH-SY5Y cells with retinoic acid and brain-derived neurotrophic factor gives rise to fully differentiated, neurotrophic factor-dependent, human neuron-like cells. J Neurochem 75:991–1003. https://doi.org/10.1046/j.1471-4159.2000.0750991.x
Fuster-Matanzo A, De Barreda EG, Dawson HN, Vitek MP, Avila J, Hernández F (2009) Function of tau protein in adult newborn neurons. FEBS Lett 583:3063–3068. https://doi.org/10.1016/j.febslet.2009.08.017
Fuster-Matanzo A, Llorens-Martín M, Jurado-Arjona J, Avila J, Hernández F (2012) Tau protein and adult hippocampal neurogenesis. Front Neurosci 6. https://doi.org/10.3389/fnins.2012.00104
Gao L, Xiao H, Ai L-Q-Y, Chen C, Lin S, Zhou Y, Ye J, Liu W (2020) Vps35 deficiency impairs Cdk5/p35 degradation and promotes the hyperphosphorylation of tau protein in retinal ganglion cells. Invest Opthalmol Vis Sci 61:1. https://doi.org/10.1167/iovs.61.1.1
Hefti MM, Kim S, Bell AJ, Betters RK, Fiock KL, Iida MA, Smalley ME, Farrell K, Fowkes ME, Crary JF (2019) Tau phosphorylation and aggregation in the developing human brain. J Neuropathol Exp Neurol 78:930–938. https://doi.org/10.1093/jnen/nlz073
Hromadkova L, Bezdekova D, Pala J, Schedin-Weiss S, Tjernberg LO, Hoschl C, Ovsepian SV (2020) Brain-derived neurotrophic factor (BDNF) promotes molecular polarization and differentiation of immature neuroblastoma cells into definitive neurons. Biochim Biophys Acta BBA - Mol Cell Res 1867:118737. https://doi.org/10.1016/j.bbamcr.2020.118737
Johnson GVW, Stoothoff WH (2004) Tau phosphorylation in neuronal cell function and dysfunction. J Cell Sci 117:5721–5729. https://doi.org/10.1242/jcs.01558
Kadavath H, Hofele RV, Biernat J, Kumar S, Tepper K, Urlaub H, Mandelkow E, Zweckstetter M (2015) Tau stabilizes microtubules by binding at the interface between tubulin heterodimers. Proc Natl Acad Sci 112:7501–7506. https://doi.org/10.1073/pnas.1504081112
Kempermann G, Gage FH, Aigner L, Song H, Curtis MA, Thuret S, Kuhn HG, Jessberger S, Frankland PW, Cameron HA, Gould E, Hen R, Abrous DN, Toni N, Schinder AF, Zhao X, Lucassen PJ, Frisén J (2018) Human adult neurogenesis: evidence and remaining questions. Cell Stem Cell 23:25–30. https://doi.org/10.1016/j.stem.2018.04.004
Lagace DC, Benavides DR, Kansy JW, Mapelli M, Greengard P, Bibb JA, Eisch AJ (2008) Cdk5 is essential for adult hippocampal neurogenesis. Proc Natl Acad Sci 105:18567–18571. https://doi.org/10.1073/pnas.0810137105
Li T, Hawkes C, Qureshi HY, Kar S, Paudel HK (2006) Cyclin-dependent protein kinase 5 primes microtubule-associated protein tau site-specifically for glycogen synthase kinase 3β. Biochemistry 45:3134–3145. https://doi.org/10.1021/bi051635j
Lowe VJ, Wiste HJ, Senjem ML, Weigand SD, Therneau TM, Boeve BF, Josephs KA, Fang P, Pandey MK, Murray ME, Kantarci K, Jones DT, Vemuri P, Graff-Radford J, Schwarz CG, Machulda MM, Mielke MM, Roberts RO, Knopman DS, Petersen RC, Jack CR (2018) Widespread brain tau and its association with ageing, Braak stage and Alzheimer’s dementia. Brain 141:271–287. https://doi.org/10.1093/brain/awx320
Masters CL, Bateman R, Blennow K, Rowe CC, Sperling RA, Cummings JL (2015) Alzheimer’s disease. Nat Rev Dis Primer 1:15056. https://doi.org/10.1038/nrdp.2015.56
Moreno-Jiménez EP, Flor-García M, Terreros-Roncal J, Rábano A, Cafini F, Pallas-Bazarra N, Ávila J, Llorens-Martín M (2019) Adult hippocampal neurogenesis is abundant in neurologically healthy subjects and drops sharply in patients with Alzheimer’s disease. Nat Med 25:554–560. https://doi.org/10.1038/s41591-019-0375-9
Niaz A, Karunia J, Mandwie M, Keay KA, Musumeci G, Al-Badri G, Castorina A (2021) Robust dopaminergic differentiation and enhanced LPS-induced neuroinflammatory response in serum-deprived human SH-SY5Y cells: implication for Parkinson’s disease. J Mol Neurosci 71:565–582. https://doi.org/10.1007/s12031-020-01678-6
Peng Y, Chu S, Yang Y, Zhang Z, Pang Z, Chen N (2021) Neuroinflammatory in vitro cell culture models and the potential applications for neurological disorders. Front Pharmacol 12:671734. https://doi.org/10.3389/fphar.2021.671734
Poewe W, Seppi K, Tanner CM, Halliday GM, Brundin P, Volkmann J, Schrag A-E, Lang AE (2017) Parkinson disease. Nat Rev Dis Primer 3:17013. https://doi.org/10.1038/nrdp.2017.13
Riegerová P, Brejcha J, Bezděková D, Chum T, Mašínová E, Čermáková N, Ovsepian SV, Cebecauer M, Štefl M (2021) Expression and localization of AβPP in SH-SY5Y cells depends on differentiation state. J Alzheimers Dis 82:485–491. https://doi.org/10.3233/JAD-201409
Rodríguez-Campuzano AG, Ortega A (2021) Glutamate transporters: critical components of glutamatergic transmission. Neuropharmacology 192:108602. https://doi.org/10.1016/j.neuropharm.2021.108602
Sahu MP, Nikkilä O, Lågas S, Kolehmainen S, Castrén E (2019) Culturing primary neurons from rat hippocampus and cortex. Neuronal Signal 3:NS20180207. https://doi.org/10.1042/NS20180207
Shipley MM, Mangold CA, Szpara ML (2016) Differentiation of the SH-SY5Y human neuroblastoma cell line. J Vis Exp: 53193. https://doi.org/10.3791/53193
Stathakos P, Jiménez-Moreno N, Crompton LA, Nistor PA, Badger JL, Barbuti PA, Kerrigan TL, Randall AD, Caldwell MA, Lane JD (2021) A monolayer hiPSC culture system for autophagy/mitophagy studies in human dopaminergic neurons. Autophagy 17:855–871. https://doi.org/10.1080/15548627.2020.1739441
Swarnkar S, Avchalumov Y, Espadas I, Grinman E, Liu X, Raveendra BL, Zucca A, Mediouni S, Sadhu A, Valente S, Page D, Miller K, Puthanveettil SV (2021) Molecular motor protein KIF5C mediates structural plasticity and long-term memory by constraining local translation. Cell Rep 36:109369. https://doi.org/10.1016/j.celrep.2021.109369
Xicoy H, Wieringa B, Martens GJM (2017) The SH-SY5Y cell line in Parkinson’s disease research: a systematic review. Mol Neurodegener 12:10. https://doi.org/10.1186/s13024-017-0149-0
Yang J-L, Lin Y-T, Chen W-Y, Yang Y-R, Sun S-F, Chen S-D (2020) The neurotrophic function of glucagon-like peptide-1 promotes human neuroblastoma differentiation via the PI3K-AKT Axis. Biology 9:348. https://doi.org/10.3390/biology9110348
Zhou Y, Wang X, Liu Y, Gu Y, Gu R, Zhang G, Lin Q (2023) Mechanisms of abnormal adult hippocampal neurogenesis in Alzheimer’s disease. Front Neurosci 17:1125376. https://doi.org/10.3389/fnins.2023.1125376
Acknowledgements
This work was directly supported by a UWE-funded Dean’s Society PhD Studentship. The authors would like to acknowledge the support of the UWE Research Technical Team in performing this study.
Author information
Authors and Affiliations
Contributions
ILT performed all of the experiments and data analysis. TJC directed the project and assisted with data analysis. LAC and MEC assisted with directing the project.
Corresponding author
Ethics declarations
Competing interests
The authors declare they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Targett, I., Crompton, L.A., Conway, M.E. et al. Differentiation of SH-SY5Y neuroblastoma cells using retinoic acid and BDNF: a model for neuronal and synaptic differentiation in neurodegeneration. In Vitro Cell.Dev.Biol.-Animal (2024). https://doi.org/10.1007/s11626-024-00948-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11626-024-00948-6