Abstract
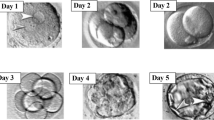
The culture of preimplantation embryos in vitro is an important method for human and mouse reproductive technology. This study aims to investigate the influence of different conditions of culture media on the preimplantation stage of mouse embryos cultured in vitro, and monitor the post-implantation development of new mice after embryo transfer to surrogate females. We demonstrated here that mouse embryos cultured in vitro in fresh M16, KSOM, Global, and HTF embryo culture media from one cell to the blastocyst stage and the subsequent embryo transfer to surrogate females are able to proceed through post-implantation development and, after birth, develop into healthy mice. However, culture of embryos in differently aged media shows various (often unpredictable) results. To find the optimal storage conditions of culture media, we suggest that the freezing and long-term storage of these media at − 80°C will not influence the quality of the media. To test this hypothesis, we grew embryos from one cell to blastocysts in vitro in the selected media after thawing and subsequently transferring them to surrogate females. Embryo culture in these four media after thawing does not affect preimplantation and postnatal mouse development. Thus, we have shown that storage of embryo culture media at low temperature (− 80°C) does not impact the quality of the media, and subsequently, it can be used for the culture of embryos for the full preimplantation period, the same as in fresh media.

Similar content being viewed by others
References
Bowman P, McLaren A (1970) Cleavage rate of mouse embryos in vivo and in vitro. J Embryol Exp Morphol 24(1):203–207
Brinster RL (1963) A method for in vitro cultivation of mouse ova from two-cell to blastocyst. Exp Cell Res 32:205–208
David A, Serr DM, Czernobilsky B (1973) Chemical composition of human oviduct fluid. Fertil Steril 24(6):435–439
Fedorov LM, Haegel-Kronenberger H, Hirchenhain J (1997) A comparison of the germline potential of differently aged ES cell lines and their transfected descendants. Transgenic Res 6(3):223–231
Guan M, Bogani D, Marschall S, Raspa M, Takeo T, Nakagata N, Fray M (2014) In vitro fertilization in mice using the MBCD-GSH protocol. Curr Protoc Mouse Biol 4(2):67–83
Hammond J Jr (1949) Recovery and culture of tubal mouse ova. Nature 163(4131):28
Harlow GM, Quinn P (1982) Development of preimplantation mouse embryos in vivo and in vitro. Aust J Biol Sci 35(2):187–193
Hogan B, Beddington R, Costantini F, Lacy E (1994) Manipulating the mouse embryo. Gold Spring Harbor Laboratory Press
Komuczki D, Stadermann A, Bentele M, Unsoeld A, Grillari J, Mueller MM, Paul A, Fischer S (2022) High cysteine concentrations in cell culture media lead to oxidative stress and reduced bioprocess performance of recombinant CHO cells. Biotechnol J 17(11):e2200029
Lippes J, Enders RG, Pragay DA, Bartholomew WR (1972) The collection and analysis of human fallopian tubal fluid. Contraception 5(2):85–103
Lippes J, Krasner J, Alfonso LA, Dacalos ED, Lucero R (1981) Human oviductal fluid proteins. Fertil Steril 36(5):623–629
Mihajlovic AI, Bruce AW (2017) The first cell-fate decision of mouse preimplantation embryo development: integrating cell position and polarity. Open Biol 7(11):170210
Moghissi KS (1970) Human fallopian tube fluid. I. Protein composition. Fertil Steril 21(12):821–829
Mohd-Fazirul M, Nor-Ashikin N, Kamsani YS, Sharaniza Ab-Rahim, Norhazlin JMY (2015) Comparison of the effects of three commercial media on preimplantation mouse embryo development and morphological grading. Biomed Res 26(3):477–484
Quinn P, Kerin JF, Warnes GM (1985) Improved pregnancy rate in human in vitro fertilization with the use of a medium based on the composition of human tubal fluid. Fertil Steril 44(4):493–498
Snyder-Keller A, Kramer LD, Zink S, Bolivar VJ (2019) Mouse strain and sex-dependent differences in long-term behavioral abnormalities and neuropathologies after developmental Zika infection. J Neurosci 39(27):5393–5403
Spangenberg E, Wallenbeck A, Eklof AC, Carlstedt-Duke J, Tjader S (2014) Housing breeding mice in three different IVC systems: maternal performance and pup development. Lab Anim 48(3):193–206
Summers MC (2013) A brief history of the development of the KSOM family of media. J Assist Reprod Genet 30(8):995–999
Tay JI, Rutherford AJ, Killick SR, Maguiness SD, Partridge RJ, Leese HJ (1997) Human tubal fluid: production, nutrient composition and response to adrenergic agents. Hum Reprod 12(11):2451–2456
Whitten WK (1956) Culture of tubal mouse ova. Nature 177(4498):96
Acknowledgements
We gratefully acknowledge Galina Fedorova for the expert technical assistance. We also thank Robert Byrd for professional animal care. We truly appreciate Thom Sounders for the helpful discussions and comments on the manuscript.
Funding
The work was supported by University Shared Resources Program at OHSU.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Marten Davenport and Yingming Wang are equal contributors.
Supplementary information
ESM 1
(DOCX 13 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Davenport, M., Wang, Y. & Fedorov, L.M. Influence of the storage conditions of embryo culture media on mouse development. In Vitro Cell.Dev.Biol.-Animal 60, 300–306 (2024). https://doi.org/10.1007/s11626-024-00884-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11626-024-00884-5