Abstract
Each 5 urothelial carcinoma (UC) cell lines with and without the v-Raf murine sarcoma virus oncogene homolog B (BRAF) gene mutation (V595E) were established and examined V595E-related tumorigenic characteristics in dogs. No typical morphological features were observed in cloned cells with and without V595E. The cell proliferation of both cloned cells showed logarithmic growth curve and those doubling time were 24.9 ± 4.1 h in V595E ( +) and 29.3 ± 11.3 h in V595E ( −). On the growth curve of xenotransplanted tumor in severe combined immunodeficiency mice, 3 out of 5 V595E ( +) and 2 out of 5 V595E ( −) cloned cells revealed gradually and remarkably increasing curve, indicating clearly tumorigenicity. The xenotransplanted tumors with V595E ( +) showed typical features of UC, such as solid proliferation of pleomorphic tumor cells, formation of papillary structure, and glandular structure. Additionally, various vascular formation was observed, probably indicating an advanced growth phase of UC. In mitogen-activated protein kinase (MAPK) signaling pathway, cytoplasmic phosphorylated-BRAF (pBRAF) and cytoplasmic and nuclear phosphorylated-ERK1/2 (pERK1/2) were detected in all 4 tumors with V595E ( +), whereas only cytoplasmic and nuclear pERK1/2 was detected in tumors with V595E ( −). Since V595E can directly activate MAPK signaling pathway, coincidence of V595E with pBRAF (phosphor Thr598/Ser601) indicates acquired resistance to BRAF inhibitors. These established UC cell lines, especially V595E ( +) cell lines, are useful tool for understanding pathophysiological states and controlling therapeutic manners of UC in dogs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Urothelial carcinoma (UC), well known as transitional cell carcinoma, is the most common tumor in the urinary tract, especially urinary bladder in dogs. The tumor, originating from urothelial epithelium cells, shows highly invasive behavior into the lamina propria and distant metastasis (Reed et al. 2012; Knapp et al. 2014; Fulkerson and Knapp 2015). Indeed, metastatic lesions were observed in 20% of dogs with UC at the time of diagnosis and in up to 50% of dogs at the death (Mutsaers et al. 2003; Allstadt et al. 2015; Mochizuki et al. 2015). Although various predispositions, such as age, breed, sex, and obesity were reported, the most important prognostic factor is metastatic dynamics of the tumor cells (Norris et al. 1992; Knapp et al. 2000; Griffin et al. 2018). As median survival time (MST) was reported by 3.5–4.0 months after the surgical treatment (Mutsaers et al. 2003; Griffin et al. 2018), similar MST (2.5–4.5 mo) was reported with the carboplatin treatment. The UC commonly shows poor response to chemotherapy and poor prognosis (Allstadt et al. 2015; Fulkerson and Knapp 2015).
Recently, the v-Raf murine sarcoma virus oncogene homolog B (BRAF) gene mutation in exon 15 (V595E), corresponding to human V600E, was identified and detected in various tumors in dogs, especially in UC with high prevalence rates of 67 to 87% (Decker et al. 2015; Mochizuki et al. 2015). It is well known that BRAF is one of the most important serine/threonine kinase and activates mitogen-activated protein kinase (MAPK) signaling pathway. The V595E can directly phosphorylate and activate its downstream MAPK kinase (MEK), followed by phosphorylated and activated extracellular single-regulated kinase (ERK1/2), indicating oncogenicity and tumorigenicity of UC as the driver mutation (Decker et al. 2015; Mochizuki et al. 2015; Mochizuki and Breen 2015). Human colorectal tumors with BRAF mutation (V600E) show histologically high grade, poor survival time, and increasing microsatellite instability and/or frequent DNA methylation related with tumorigenicity (Nagasaka et al. 2004; Li et al. 2006; Jung et al. 2021). In contrast, our report demonstrated that V595E coincided with phosphorylated BRAF (pBRAF), indicating key of the resistance to BRAF inhibitors, in all 13 formalin-fixed tissue samples of UC with V595E ( +) (Yamasaki et al. 2022).
On the other hand, tumor cell lines are one of the most valuable tools for understanding morphological differentiation, mechanism of cell growth, biological behavior, tumorigenicity of neoplastic cells, and responses with anti-tumor drugs (Choi et al. 2014; Kito et al. 2018; Packeiser et al. 2020). However, there are few reports on cell lines established from original UC with V595E ( +) in dogs (Jung et al. 2021). In the present study, each 5 cell lines from UC with and without V595E were established and examined on morphological features, cell proliferation, tumorigenicity, and expression of phosphorylated-BRAF and phosphorylated-ERK1/2 in xenotransplanted tumors.
Materials and methods
Clinical characteristics of original tumor samples for establishment of UC cell lines
A total of 10 tissue samples of UC, including 2 catheter-aspiration specimens, were used. All tissue samples confirmed diagnosis or strongly suspected, based on their histopathological or cytological findings, by 2 board-certified veterinary pathologists at the Japanese College of Veterinary Pathologists. The UC tissue samples were subdivided into each 5 cases of BRAF mutation with and without V595E, abbreviated as V595E ( +) and V595E ( −), respectively. The V595E ( −) means wild-type gene of BRAF. Clinical characteristics of these samples are represented on breed, age, sex, and histopathological or cytological findings in Fig. 1. Most of UC tissue samples revealed some typical histopathological or cytological features of UC, such as proliferation of neoplastic cells in papillary shape, in oval cells with anisocytosis, and in gland-like formation. There were no remarkable differences of clinical characteristics between V595E ( +) and V595E ( −) with an exception of the sex. In V595E ( +), 4 out of 5 cases were neutered female dogs, and the remaining was neutered male, whereas one intact male, two neutered male, one intact female, and one neutered female were observed in V595E ( −).
Clinical characteristics of tissue samples for establishment of cell lines from urothelial carcinoma (UC) with and without BRAF mutation (V595E) in dogs. Four breeds, consisted of 2 miniature Dachshund, Siberian Husky, Cairn terrier, and Maltese, are observed in V595E ( +) cases, whereas 4 breeds, consisted of 2 miniature Dachshund, Shih Tzu, Shetland sheepdog, and French bulldog, are observed in V595E ( −). The age ranged from 9 years and 9 months old to 13 years and 7 months old in V595E ( +) and from 8 years old to 13 years and 3 months old in V595E ( −). Gender of each 5 cases of V595E ( +) and V595E ( −) is 4 neutered female and 1 neutered male dog, and 1 neutered female, 1 intact female, 2 neutered male, and 1 intact male dog, respectively. There are no remarkable differences on histological or cytological features between V595E ( +) and V595E ( −) samples. Both catheter-aspirations of V595E ( +) and V595E ( −) reveal atypical epithelial cells in oval shape and proliferation of tumor cells in papillary shape with anisocytosis (TCCV-Ka, TCC-Ii). Proliferation of tumor cells in sheet formation (TCCV-So, TCC-Sa), various atypical epithelioid cells (TCCV-Ec, TCC-Ue), in an island form (TCCV-Ni), and in papillary form (TCCV-Ic, TCC-Yo, TCC-Oh) are observed
Analysis of BRAF mutation (V595E)
The V595E mutation was analyzed by polymerase chain reaction-restriction fragment length polymorphism (PCR–RFLP) (Decker et al. 2015; Mochizuki et al. 2015; Jung et al. 2021; Yamasaki et al. 2022). Using primer pairs, reverse primer 5′-TGG CCT CAA TTC TTA CCA TCC AC-3′, designed by Decker et al. (2015), and forward primer 5′-GTA ATG CTT GCT TTG CTA GGA 3′, originally designed from genomic DNA, based on GenBank (accession No. NC_006598.2), 196 base pair (bp) DNA fragment, corresponding to human BRAF gene exon 15, was amplified (Yamasaki et al. 2022). When V595E with substitution of nucleotide (c.1784 T > A), corresponding to human V600E with substitution of c.1799 T > A, was developed, the location of restriction enzyme site with BtsIMutl (New England Biolabs Japan Inc., Tokyo, Japan), sequencing CAGTG, disappeared (Fig. 2A, B). As PCR amplicons of wild-type BRAF gene were digested by BtsIMutI, V595E was clearly detected by RFLP analysis. Briefly, the amplicon of wild-type BRAF gene is digested into 2 DNA fragments of 122 and 74 bp, whereas BRAF mutant gene (V595E) is not digested, indicating 3 fragments (196, 122, and 74 bp) (Fig. 2C).
Genomic DNA sequence, PCR-sequencing chromatogram, and PCR–RFLP electropherogram of BRAF exon 15 gene with mutation (V595E) and wild type. (A) DNA sequence of wild type (WT) of BRAF gene in human (NM_004333.4) (upper 1st line) and in dog (XM_005629550.1) (2nd line). Using primer pairs mentioned in the “Materials and methods” section, the sequences are highly conserved between human and dogs (Nos. 1–3), although 2 silent point mutations are observed (green band). DNA fragment of 196 base pair (bp), corresponding to human BRAF exon 15 gene, is amplified and sequenced. Squared 5 genomic bases, in which latter 3 bases consist of codon 595 and include mutant sequence (red character; 6th line), are the digestion site of BtsIMutI restriction enzyme. (B) Chromatogram of the wild type (left) and mutant (right) sequence of BRAF in dogs. When V595E with substitution of c.1784 T > A, corresponding to human V600E with substitution of c.1799 T > A, is developed, the restriction enzyme site, sequencing CAGTG, disappeared. (C) V595E is clearly detected on PCR–RFLP electropherogram. The amplicons are digested to 2 fragments of 122 and 74 bp in wild-type sequence, whereas amplicon is not digested in mutant sequence, demonstrating 3 bands of 196-, 122-, and 74-bp fragment
Establishment of cell lines (cell culture and cloning)
The tissue or catheter aspiration samples were cut, minced, dissolved, and seeded in 90-mm tissue culture dish (Nunclon, Thermo Fisher Scientific, Waltham, MA). The tumor cells were maintained in a humidified atmosphere 5% CO2 at 37 °C in Dulbecco’s modified Eagle’s medium (Gibco, Thermo Fisher Scientific) supplemented with 20% fetal bovine serum (Gibco, Thermo Fisher Scientific) and 1% penicillin–streptomycin (Sigma, Sigma-Aldrich, Saint Louis, MO). The cells were passaged when they reached confluence. After 50–60 passage cultivation, cloned cells were obtained by the limiting dilution culture
Cell proliferation assay (doubling time)
Cell proliferation assay was performed for calculating of the doubling time. The cloned tumor cells from UC with V595E ( +) and V595 ( −) were cultured in 12-well culture plate (Falcon, Corning Incorporated Life Sciences, Tewksbury, MA) at 1.5 × 105 cells/well to obtain the growth curve. The cells were counted on hemocytometer of every day in triplicate wells from day 1 to day 5.
Xenotransplantation of the cloned UC cells into SCID mice
Each 5 cloned tumor cells with V595E ( +) and V595E ( −) were subcutaneously inoculated with 0.22 − 8.0 × 107 cells in 200 μl of physiological saline (TERUMO Inc, Tokyo, Japan) into the lower flunk of each 3 female 5-wk-old severe combined immunodeficiency (SCID) mice (total 30 mice) (FOX CHASE SCID CB-17/lcr-scid/scidJcl; CLEA Japan Inc., Tokyo, Japan). The inoculated mice were housed in a cage (3 mice/cage) with free access to water and a standard purified rodent growth diet (AIN-93G, Oriental Yeast, Tokyo, Japan). When the mass was palpable at the inoculation site, designed as day 0 after the inoculation in this experiment, the tumor volume (length × width × height) was measured every day, using a digital vernier caliper. At the 28th–35th day after the inoculation, the mice were euthanized with isoflurane and the tumor was resected for histopathological and immunohistochemical examinations.
All animal experiments were approved by the Committee of Animal Experiments, Graduate School of Agricultural and Life Sciences, The University of Tokyo (approve Number P17-017).
Histopathological and immunohistochemical analysis for xenotransplanted tumor
For histopathological analysis, total 9 xenotransplanted tumor samples, consisted of 4 with V595E ( +) and 5 with V595E ( −) tumor tissues, were fixed in 10% neutral buffered formalin, routinely paraffin processed, sectioned at 4 μm, and stained with hematoxylin and eosin (H&E). For immunohistochemical analysis of phosphorylated-BRAF (pBRAF) and phosphorylated-ERK1/2 (pERK1/2), 4-μm tissue sections of xenotransplanted tumor were used. Briefly, antigen retrieval was performed by autoclaving tissue sections in citrate buffer (pH 6.0) at 121 °C for 10 min. Endogenous peroxidase activity in the tissue sections was inactivated with 3% hydrogen peroxide in methanol at room temperature for 5 min. To block nonspecific reactions, the sections were incubated with 8% skimmed milk in Tris-buffered saline (TBS) at 37 °C for 30 min. The sections were then incubated at 4 °C overnight with each primary antibody: rabbit polyclonal anti-BRAF (phosphor Thr598/Ser601) (Cat. GTX85596, 1:100, Gene Tex Inc., Irvine, CA) and rabbit monoclonal anti-phospho-p44/42 MAPK (Erk 1/2) (Thr 202/Tyr204) (20G11) (Cat. #4376S, 1:400, CST Japan, Tokyo, Japan). The primary antibodies were replaced with TBS to produce a negative control. After 3 washings with TBS, the sections were treated with Dako EnVision + System-horseradish peroxidase-labeled polymer anti-rabbit secondary antibodies (Agilent Technologies Japan Ltd, Tokyo, Japan) at 37 °C for 40 min. The chromogen consisted of 0.05% 3–3′-diaminobenzidine and 0.03% hydrogen peroxide in Tris–HCl buffer. The sections were counterstained with Mayer’s hematoxylin.
Results
Establishment of cell lines (cell culture and cloning)
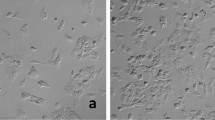
Figure 3 shows inverted microscopic images of both primary cultured cells (passage number: 3–5) and cloned cells (cloned by the limiting dilution method) of UC with V595E ( +) (1st and 2nd column, respectively) and with V595E ( −) (3rd and 4th columns, respectively). The primary cultured cells from UC with V595E ( +) consisted of spindle cells (fibroblast-like cells), oval to round cells (epithelial-like cells), and sometimes polygonal and giant cells. In addition, those from UC with V595E ( −) revealed island formation (2 cases), and consisted of spindle cells and oval to round cells. In cloned cells, there were no remarkable morphological differences between V595E ( +) and V595E ( −) cells, such as round to oval cells (epithelial-like cells) and sometimes with large polygonal cells. Morphological features (cell shape) are summarized in Table 1.
Primary cultured cells and cloned cells from UC with V595E ( +) and V595E ( −). Inverted microscopic images of primary cultured cells and cloned cells from UC with V595E ( +) are represented in the 1st and the 2nd columns, respectively, and those with V595E ( −) are represented in the 3rd and the 4th columns, respectively. The primary cultured cells with V595E ( +) are grown in adherent and monolayer cells with spindle and round cell shape (TCCV-Ka), round and large polygonal cell shape (TCCV-So, TCCV-Ec), large polygonal cell shape with polynuclear (TCCV-Ni), and round and large polygonal cell shape (TCCV-Ic), whereas the cells with V595E ( −) are grown in island formation (TCC-Ii, TCC-Yo), round to oval cell shape (TCC-Oh), and spindle cell shape (TCC-Sa, TCC-Ue). The cloned cells with V595E ( +) are grown round to oval and large polygonal cell shape (TCCV-Ka), round to oval cell shape (TCCV-So), round cell shape (TCCV-Ec), round and squamous cell shape (TCCV-Ni), and squamous and large polygonal cell shape (TCCV-Ic), whereas the cells with V595E ( −) are grown in round to oval cell shape (TCC-Ii), spindle-like and large polygonal cell shape (TCC-Yo), squamous and large polygonal cell shape (TCC-Oh), and squamous cell shape (TCC-Sa, TCC-Ue). There are no remarkable morphological differences in cloned cells between V595E ( +) and V595E ( −). Bar = 20 μm
Cell proliferation assay (doubling time)
Both cloned cells from UC with V595E ( +) and V595E ( −) showed similar logarithmic growth curve (Fig. 4A; left and right graph, respectively). Those calculating doubling time are summarized in Fig. 4 B. The doubling time of the cloned cells with V595E ( +) and V595E ( −) were 24.9 ± 4.1 h and 29.3 ± 11.3 h (average ± SD), respectively.
Cell proliferation assay (doubling time) of the cloned cells from UC with V595E ( +) and V595E ( −). Each 5 cloned cells from both UC with V595E ( +) and V595E ( −) show similar logarithmic cell growth (A: left and right graph, respectively). Calculating those doubling time are summarized in the table (B)
Xenotransplantation of the cloned UC cells into severe combined immunodeficiency mice
There were 3 types of growth curve, such as gradually and remarkably increasing, approximately maintaining tumor size at day 0, and gradually decreasing, in xenotransplanted tumors. The SCID mice, inoculated with 3 cloned cells with V595E ( +), showed gradually and remarkably increasing curve. One of the remaining 2 SCID mice revealed approximately maintaining curve and the other mice revealed gradually decreasing curve by day 14 (Fig. 5, left graph), whereas 2 SCID mice, inoculated with cloned cells with V595E ( −), showed gradually and remarkably increasing curve. The remaining 3 SCID mice revealed approximately maintaining curve (Fig. 5, right graph).
Tumor growth curve of cloned UC cells with V595E ( +) or V595E ( −) in subcutaneously xenotransplanted into SCID mice. There are 3 types of tumor growth curve, such as gradually and remarkably increasing tumor size, approximately maintaining tumor size at day 0, and gradually decreasing in tumor size. The SCID mice, inoculated with 3 out of 5 cloned cells with V595E ( +), show gradually and remarkably increasing tumor size (TCCV-Ka, TCCV-So, and TCCV-Ec). One of the remaining 2 SCID mice, TCCV-Ni reveals approximately maintaining tumor size at day 0 and another TCCV-Ic reveals decreasing in tumor size by day 14 (left graph), whereas 2 out of 5 SCID mice, inoculated with cloned cells with V595E ( −), show gradually and remarkably increasing tumor size (TCC-Ii and TCC-Yo). The remaining 3 SCID mice (TCC-Oh, TCC-Sa, and TCC-Ue) reveal maintaining tumor size (right graph)
Histological and immunohistochemical features of xenotransplanted tumor, inoculated cloned cells with V595E ( +) or V595E ( −)
With an exception of the decreasing case, 4 xenotransplanted tumors, inoculated cloned cells with V595E ( +), showed some typical histopathological features of UC, such as formation of neoplastic foci, solid proliferation of pleomorphic tumor cells, formation of papillary structure toward the lumen, and glandular structure with tumor cells. In addition, various vascular formation was observed in 3 cases (Fig. 6, left column). Cytoplasmic immunoreactivity against pBRAF (phosphor Thr598/Ser601) was detected in all 4 cases examined. Although nuclear immunoreactivity was detected in 2 cases, these reactivities were non-specific, due to pBRAF being limited in cytoplasm (center column). Cytoplasmic immunoreactivities against pERK1/2 (Thr 202/Tyr204), associated with nuclear immunoreactivity, were detected in all 4 cases, indicating activation of MAPK signaling pathway (right column).
Histopathological futures and expression of phosphorylated-BRAF (pBRAF) and phosphorylated-ERK1/2 (pERK1/2) in xenotransplanted tumors with V595E ( +) cloned cells. The 4 cases of xenotransplanted tumors, with the exception of decreasing case (TCCV-Ic), reveal some typical histopathological features of UC, such as the formation of neoplastic foci (TCCV-Ka and TCCV-So), formation of glandular structure with tumor cells (TCCV-Ec), and solid proliferation of pleomorphic tumor cells (TCCV-Ni). Additionally, various vacuoles are observed in 3 cases (TCCV-Ka, TCCV-So, and TCCV-Ni) (left column; H&E staining, Bar = 50 μm). On the results of immunohistochemistry, cytoplasmic immunoreactivity against pBRAF is detected in all 4 cases examined. Although nuclear immunoreactivity is detected in 2 cases (TCCV-Ka and TCCV-Ec), these reactivities are non-specific, due to pBRAF being limited in cytoplasm (center column, bar = 50 μm). Additionally, cytoplasmic immunoreactivities against pERK1/2 are detected in all 4 cases with positive reactions of nuclear immunoreactivity (right column, bar = 50 μm)
Figure 7 shows xenotransplanted tumors, inoculated with cloned cells of V595E ( −). All 5 tumors revealed typical histopathological features of UC, such as the proliferation of the pleomorphic tumor cells in sheet formation, sometimes in gland-like formation, formation in papillary structure toward the lumen, formation in island shape with thin septum, and formation in glandular structure (left column). None of positive cytoplasmic immunoreactivity against pBRAF was detected, whereas cytoplasmic immunoreactivity against pERK1/2 was detected in 2 cases (center and right column, respectively).
Histopathological futures and expression of phosphorylated-BRAF (pBRAF) and phophorylated-ERK1/2 (pERK1/2) in xenotransplanted tumors with V595E ( −) cloned cells. All 5 tumors reveal typical histopathological features of UC, such as proliferation of the pleomorphic tumor cells in sheet formation (TCC-Ii) and sometimes in gland-like formation (TCC-Ue), papillary structure toward the lumen (TCC-Yo), formation in island shape with thin septum (TCC-Oh), and formation in glandular structure (TCC-Sa) (left column, H&E staining bar = 50 μm). None of positive cytoplasmic immunoreactivity against pBRAF is detected, whereas cytoplasmic immunoreactivity against pERK1/2 is detected in 2 cases (TCC-Sa, TCC-Ue) (center and right column, respectively, bar = 50 μm)
Discussion
It is widely accepted that V595E is the most important oncogenic mutations of BRAF gene in dogs, corresponding to V600E in human tumors. The V595E is the driver mutation of canine UC with extremely high prevalence rates and activates MAPK signaling pathway, indicating tumorigenicity of UC (Decker et al. 2015; Mochizuki et al. 2015). The PCR–RFLP analysis, as shown in this study, is available tool for detection of V595E, providing genomic DNA sequence and PCR sequencing chromatogram of V595E. The V595E is closely associated with morphology, cell growth, tumorigenesis, metastatic mechanism, and potential of chemotherapy of UC, like as reported in the tumor with V600E (Garnett and Marais 2004; Li et al. 2006; Jung et al. 2021). Tumor cell lines are valuable tool for understanding cell growth, biological behavior, tumorigenicity, and responses with anti-tumor drugs (Choi et al. 2014; Kito et al. 2018; Packeiser et al. 2020).
Although 15 cell lines of canine UC are listed in bladder cancer (Dhawan et al. 2009; Cekanova et al. 2013; Decker et al. 2015; Zuiverloon et al. 2018), only 2 cell lines are established from original tumors with V595E ( +) and examined their characteristics (Jung et al. 2021). In this study, each 5 newly cell lines from UC with and without V595E were established. Both primary cultured cells with and without V595E revealed adherent monolayer cells and sometimes island formation. They revealed heterogeneous cell in shape, such as fibroblast-like cells, epithelial-like cells, round cell, and sometimes giant cells, probably because originating parent tumor consisted of different growth phase of cells (Palyi et al. 1977; Jung et al. 2021). There were no typical morphological features related with V595E of cloned cells.
In cell proliferation, both cloned cells with and without V595E showed similar logarithmic growth curve and doubling time, calculating as 24.9 ± 4.1 h in V595E ( +) and 29.3 ± 11.3 h in V595E ( −). Although slightly shorter doubling time (ranged 17.7–20.0 h) was reported in UC with V595E ( +), these values were overlapped with wide range of doubling time in UC with V595E ( −) (Dhawan et al. 2009; Zuiverloon et al. 2018; Jung et al. 2021). In growth curve of xenotransplanted tumor, subcutaneously inoculated with both cloned cells with and without V595E into SCID mice, 3 out of 5 tumors with V595E ( +) and 2 out of 5 tumors with V595E ( −) revealed gradually and remarkably increasing of tumor size, indicating strong tumorigenic activity. As the tumorgenicity is closely related to invasion and metastasis, poor prognosis commonly observed in UC might be induced by their activity (Norris et al. 1992; Knapp et al. 2000; Griffin et al. 2018). On the other hand, one xenotransplanted tumor with V595E ( +) gradually decreased during the experimental period; like as those in previous reports. Tumor formation required some interaction with fibroblasts, macrophages, and adjacent connective tissues (Dhawan et al. 2009; Rathore and Cekanova 2014; Jung et al. 2021). Xenotransplanted tumors with V595E ( +) showed some typical histopathological features of UC, such as the formation of neoplastic foci, solid proliferation, and formation of papillary structure and/or glandular structure. Various vascular formation was observed in 3 out of 4 tumors. Whereas all 5 xenotransplanted tumors with V595E ( −) also revealed typical histopathological features of UC, such as sheet formation, sometimes in gland-like formation, papillary structure, and island in shape, these features might be reflected early growing stage of UC (Li et al. 2006; Patrick et al. 2006; Grassinger et al. 2019).
In MAPK signaling pathway, all 4 tumors with V595E ( +) revealed cytoplasmic immunoreactivity against pBRAF (phosphor Thr598/Ser601). Nuclear immunoreactivity against pBRAF was also detected in 2 tumors; however, nuclear reactivities were non-specific, due to pBRAF being fundamentally limited in cytoplasm (Davies et al. 2002; Loo et al. 2018). In contrast, no cytoplasmic pBRAF immunoreactivity was detected in all tumors with V595E ( −). Phosphorylation sites at Thr598/Ser601 in BRAF are major and essential sites of the activated RAS (rat sarcoma viral oncogene homolog) signaling, consisting of RKT (receptor tyrosine kinase)-RAS-BRAF-MEK-ERK cascade (Zhang and Guan 2000, 2001). It is well known that V595E, like as other BRAF mutations, can directly phosphorylate and activate MEK with subsequent activation of ERK1/2, by changing of the conformation in activating loop of BRAF (Liu et al. 2019; Maloney et al. 2021). In all 4 tumors with V595E ( +), both cytoplasmic and nuclear immunoreactivity against pERK1/2 (phosphor-p44/42) were detected, indicating tumorigenicity of the tumor. As nuclear pERK1/2, translocated from cytoplasm, indicates progressive signaling process in MAPK pathway, UC with V595E ( +) might be growing stage of the tumor (Parikh et al 2012; Maik-Rachline et al 2019).
Interestingly, UC with V595E ( +) constantly shows pBRAF, as the same in our previous study in formalin fixed 13 UC tissue samples (Yamasaki et al. 2022) and in 2 cell lines reported by Jung et al. (2021). The BRAF gene mutation (V600E) never coincides with activated RAS and RTK, both of which induces pBRAF, in human tumors (Haling et al. 2014; Mochizuki and Breen 2017; Dvorak et al. 2019). In contrast, many researchers reported that pBRAF, detected in the melanoma with acquired resistant to BRAF inhibitors, is caused by paradoxically activation of upstream RTK or RAS through ERK1/2 feedback mechanism, suggesting new strategies for UC chemotherapy (Nazarian et al. 2010; Little et al. 2011; Prahallad et al. 2012; Holderfield et al. 2013). When activation of MAPK signaling pathway as pERK1/2 without pBRAF was observed in UC with V595E ( −), activation of RTK-RAS-PI3K (phosphatidylinositol-3-kinase)-mTOR (mammalian target of rapamycin) cascade, or GPCR (G protein–coupled receptor)-GNAG (G protein: guanine nucleotide binding protein)-MEK-ERK1/2 cascade might be related with tumorigenicity of UC (Porta et al. 2014; Thapa et al. 2018; Lee et al. 2020).
Conclusion
Each 5 cloned cells from UC with and without BRAF gene mutation (V595E) were established and examined V595E-related tumorigenic characteristics in dogs. No typical morphological features were observed in cloned cells with and without V595E. Both cell proliferation rate (doubling time) and tumorigenicity (xennotransplanted tumor growth) in cloned cells with V595E ( +) were similar to those with V595E ( −). The xenotransplanted tumors with V595E ( +) revealed typical features of UC and expressed nuclear pERK1/2, which probably indicate advanced stage of tumor growth. Coincidence of V595E with pBRAF is an important factor for acquired resistance to BRAF inhibitors. These established UC cell lines, especially V595E ( +) cell lines, are useful tool for understanding of pathophysiological states and controlling of therapeutic manners of UC in dogs.
References
Allstadt SD, Roddriguez CO Jr, Boostrom B, Rebhum RB, Skorupski KA (2015) Randomized phase III trial of piroxicam in combination with mitoxantrone or carboplatin for first-line treatment of urogenital tract transitional cell carcinoma in dogs. J Vet Intern Med 29:261–267
Cekanova M, Uddin MD, Bartges JW, Callens A, Legendre AM, Rathore K, Wright L, Carter A, Marnett LJ (2013) Molecular imaging of cyclooxygenase-2 in canine transitional cell carcinomas in vitro and in vivo. Cancer Prev Res (phila) 6(5):466–476
Choi S-J, Lee H, Choe C, Shin Y-S, Lee J, Moon S-H, Kim J (2014) Establishment and characterization of a lung cancer cell line, SMC-L001, from a lung adenocarcinoma. In Vitro Cell Dev Biol-Animal 50:519–526
Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, Teague J, Woffendin H, Garnett MJ, Bottomley W, Davis N, Dicks E, Ewing R, Floyd Y, Gray K, Hall S, Hawes R, Hughes J, Kosmidou V, Menzies A, Mould C, Parker A, Jtevens CS, Watt S, Hooper S, Wilson R, Jayatilake H, Gusterson BA, Cooper C, Shipley J, Hargrave D, Pritchard-Jones K, Maitland N, Chenevix-Trench G, Riggins GJ, Bigner DD, Palmieri G, Cossu A, Flanagan A, Nicholson A, Ho JWC, Leung SY, Yuen ST, Weber BL, Seigler HF, Darrow TL, Paterson H, Marais R, Marshall CJ, Wooster R, Stratton MR, Futreal PA (2002) Mutation of the BRAF gene in human cancer. Nature 417:949–954
Decker B, Parker HG, Dhawan D, Kwon EM, Karlins E, Davis DW, Ramos-Vara JA, Bonney PL, McNiel EA, Knapp DW, Ostrander EA (2015) Homologous mutation to human BRAF V600E is common in naturally occurring canine bladder cancer – evidence for a relevant model system and urine-based diagnostic test. Mol Cancer Res 13(6):993–1002
Dhawan D, Ramos-Vara JA, Stewart JC, Zheng R, Knapp DW (2009) Canine invasive transitional cell carcinoma cell lines: in vitro tools to complement a relevant animal model of invasive urinary bladder cancer. Urol Oncol 27:284–292
Dvorak K, Higgins A, Palting J, Cohen M, Bruuhoeber P (2019) Immunohistochemistry with anti-BRAF V600E (VE1) mouse monoclonal antibody is a sensitive method for detection of the BRAF V600E mutation in colon cancer: evaluation of 120 cases with and without KRAS mutation and literature review. Pathol Oncol Res 25:349–359
Fulkerson CM, Knapp DW (2015) Management of transitional cell carcinoma of the urinary bladder in dogs: A review. Vet J 205:217–225
Garnett MJ, Marais R (2004) Guilty as charged: B-RAF is a human oncogene. Cancer Cell 6:313–319
Grassinger JM, Merz S, Aupperle-Lellbach H, Erhard H, Klopfleish R (2019) Correlation of BRAF variant V595E, Breed, histological grade and cyclooxygenase-2 expression in canine transitional cell carcinoma. Vet Sci 6:31. https://doi.org/10.3390/vetsci6010031
Griffin MA, Culp WTN, Rebhun RB (2018) Lower urinary tract neoplasia. Vet Sci 5:96. https://doi.org/10.3390/vetsci5040096
Haling JR, Sudhamsu J, Yen I, Sideris S, Sandoval W, Phung W, Bravo BJ, Giannetti AM, Peck A, Masselot A, Morales T, Smith D, Brandhuber BJ, Hymowitz SG, Malek S (2014) Structure of the BRAF-MEK complex reveals a kinase activity independent role for BRAF in MAPK signaling. Cancer Cell 26:402–413
Holderfield M, Merritt H, Chan J, Wallroth M, Tandeske L, Zhai H, Tellew J, Hardy S, Hekmat-Nejad N, Stuart DD, McCormick F, Nagel TE (2013) RAF inhibitors activate the MAPK pathway by relieving inhibitory autophosphorylation. Cancer Cell 23:594–602
Jung H, Bae K, Lee JY, Kim J-H, Han H-J, Yoon H-Y, Yoon K-A (2021) Establishment of canine transitional cell carcinoma cell lines habouring BRAF V595E mutation as a therapeutic target. Int J Mol Sci 22:9151. https://doi.org/10.3390/ijms22179151
Kito F, Oyama R, Sakumoto M, Takahashi M, Shiozawa K, Qiao Z, Sakamoto H, Hirose T, Setsu N, Yoshida A, Kawai A, Kondo T (2018) Establishment and characterization of novel patient-drived osteosarcoma xenograft and cell line. In Vitro Cell Dev Biol-Animal 54:528–536
Knapp DW, Glickman NW, DeNicola DB, Bonney PL, Lin TL, Glickman LT (2000) Naturally-occurring canine transitional cell carcinoma of the urinary bladder: a relevant model of human invasive bladder cancer. Uro Oncol 5:47–59
Knapp DW, Ramos-Vera JA, Moore GE, Dhawan D, Bonney PL, Young KE (2014) Urinary bladder cancer in dogs, a naturally occurring model for cancer biology and drug development. ILAR J 55(1):100–118
Lee S, Rauch J, Kolch W (2020) Targeting MAPK signaling in cancer: mechanisms of drug resistance and sensitivity. Int J Mol Sci 21:1102. https://doi.org/10.3390/jms21031102
Li WQ, Kawakami K, Ruszkiewicz A, Bennett G, Moor J, Iacopetta B (2006) BRAF mutations are associated with clinical, pathological and molecular features of colorectal cancer independently of microsatellite instability status. Mol Cancer 5:2. https://doi.org/10.1186/1476-4598-5-2
Little AS, Balmanno K, Sale MJ, Newman S, Dry JR, Hampson M, Edwards PAW, Smith PD, Cook SJ (2011) Amplification of the driving oncogene, KRAS or BRAF, underpins acquired resistance to MEK1/2 inhibitors in colorectal cancer cells. Sci Signal 4(166):ra 17. https://doi.org/10.1126/scisignal2001752
Liu T, Wang Z, Guo P, Ding N (2019) Electrostatic mechanism of V600E mutation-induced B-Raf constitutive activation in colorectal cancer: molecular implications for the selectivity difference between type-I and type-II inhibitors. Eur Biophys J 48:73–82
Loo E, Khalili P, Beuhler K, Siddiqi I, Vasef MA (2018) BRAF V600E mutation across multiple tumor types: correlation between DNA-based sequencing and mutation-specific immunohistochemistry. Appl Immunohistochem Mol Morphophol 26:709–713
Maik-Rachline G, Hacohen-Lev-Ran A, Seger R (2019) Nuclear ERK: mechanism of translocation, substrates, and role in cancer. Int J Mol Sci 20:1194. https://doi.org/10.3390/ijms20051194
Maloney RC, Zhang M, Jang H, Nussinov R (2021) The mechanism of activation of monomeric B-Raf V600E. Comput Struct Biotechnol J 19:3349–3363
Mochizuki H, Breen M (2015) Comparative aspects of BRAF mutations in canine cancers. Vet Sci 2:231–245
Mochizuki H, Breen M (2017) Sequence analysis of RAS and RAF mutation hot spots in canine carcinoma. Vet Comp Oncol 15(4):1598–1605
Mochizuki H, Kennedy K, Shapiro SG, Breen M (2015) BRAF mutation in canine cancers. PLoS ONE 10(6):e0129534. https://doi.org/10.1371/journal.pone.0129534
Mutsaers AJ, Widmer WR, Knapp DW (2003) Canine transitional cell carcinoma. J Vet Intern Med 17:136–144
Nagasaka T, Sasamoto H, Notohara K, Culling HM, Takeda M, Kimura K, Kambara T, MacPhee DG, Yaung J, Leggett BA, Jass JR, Tanaka N, Matsubara N (2004) Colorectal cancer with mutation in BRAF, KRAS, and wild-type with respect to both oncogenes showing different patterns of DNA methylation. J Clin Oncol 22:4584–4594
Nazarian R, Shi H, Wang QL, Kong X, Koya RC, Lee H, Chen Z, Lee M-K, Attar N, Sazegar H, Chodon T, Nelson SF, McArthur G, Sosman JA, Ribas A, Lo RS (2010) Melanomas aquire resistance to B-RAF (V600E) inhibition by RTK or N-RAS upregulation. Nature 468:973–977
Norris AM, Laing EJ, Valli VEO, Withrow SJ, Macy DW, Ogilvie GK, Tomlinson J, McCaw D, Pidgeon G, Jacobs RM (1992) Canine bladder and urethral tumors: a retrospective study of 115 cases (1980–1985). J Vet Intern Med 6:145–153
Packeiser E-M, Hewicker-Trautwein M, Thiemeyer H, Mohr A, Junginger J, Schille JT, Escobar HM, Nolte I (2020) Characterization of six canine prostate adenocarcinoma and three transitional cell carcinoma cell lines derived from primary tumor tissues as well as metastasis. PLoS ONE 15(3):e0230272. https://doi.org/10.1371/journal.pone.0230272
Palyi I, Olah E, Sugar J (1977) Drug sensitivity study on glonal cell lines isolated from heteroploidy tumour cell populations. I. Dose response of clones growing in monolayer cultures. Int J Cancer 19:859–865
Parikh N, Shuck RL, Nguyen T-A, Herron A, Donehower LA (2012) Mouse tissues that undergo neoplastic progression after K-Ras activation are distinguished by nuclear translocation of phosphor-ERK1/2 and robust tumor suppressor responses. Mol Cancer Res 10(6):845–855
Patrick DJ, Fitzgerald SD, Sesterhenn IA, Davis CJ, Kiupel M (2006) Classification of canine urinary bladder urothelial tumours based on the World Health Organization/ International Society of Urological Pathology Consensus Classification. J Comp Path 135:190–199
Porta C, Paglino C, Mosca A (2014) Targeting PI3K/AKT/mTOR signaling in cancer. Front Oncol 4.https://doi.org/10.3389/fonc.2014.00064
Prahallad A, Sun C, Huang S, Nicolantonio FD, Salazar R, Zecchin D, Beijersbergen RL, Bardelli A, Bernards R (2012) Unresponsiveness of colon cancer to BRAF (V600E) inhibition through feedback activation of EGFR. Nature 483:100–104
Rathore K, Cekanova M (2014) Animal model of naturally occurring bladder cancer: characterization of four new canine transitional cell carcinoma cell lines. BMC Cancer 14: 465. http://www.biomedcentral.com/1471-2407/14/465
Reed LT, Knapp DW, Miller MA (2012) Cutaneous metastasis of transitional cell carcinoma in 12 dogs. Vet Pathol 50(4):676–681
Thapa D, Stoner MW, Zhang M, Xie B, Manning JR, Guimaraes D, Shiva S, Jurczak MJ, Scott S (2018) Adropin regulates pyruvate dehydrogenase in cardiac cell via novel GPCR-MAPL-PDK4 signaling pathway. Redox Biol 18:25–32
Yamasaki H, Uematsu Y, Hayashi Y, Yamashita M, Tei M, Uchida K, Ono K, Hirao H (2022) Coincidence of v-raf murine sarcoma viral oncogene homolog B mutation (V595E) with phosphorylated v-raf murine sarcoma viral oncogene homolog B in urothelial carcinoma in dogs. Can J Vet Res 86(4):286–293
Zhang B-H, Guan K-L (2000) Activation of B-Raf kinase requires phosphorylation of the conserved residues Thr598 and Ser601. EMBO J 19(20):5429–5439
Zhang B-H, Guan K-L (2001) Regulation of the Raf kinase by phosphorylation. Exp Lung Res 27:269–295
Zuiverloon TCM, de Jong FC, Costello JC, Theodoreseu D (2018) Systemic review: Characteristics and preclinical uses of bladder cancer cell lines. Bladder Cancer 4:169–183
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted.
I already described in manuscript, “The inoculated mice were housed in a cage (3 mice/cage) with free access to water and a standard purified rodent growth diet (AIN-93G, Oriental Yeast, Tokyo, Japan). When the mass was palpable at the inoculation site, designed as day 0 after the inoculation in this experiment, the tumor volume (length x width x height) was measured every day. At the 28-35th day after the inoculation, the mice were euthanized with isoflurane and the tumor was resected for histopathological and immunohistochemical examinations.
All animal experiments were approved by the Committee of Animal Experiments, Graduate School of Agricultural and Life Sciences, The University of Tokyo (approve Number P17-017).”
Conflict of interest
The authors declare no competing interests.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yamasaki, H., Uematsu, Y., Okano, K. et al. Establishment and characterization of urothelial carcinoma cell lines with and without BRAF mutation (V595E) in dogs. In Vitro Cell.Dev.Biol.-Animal 58, 898–911 (2022). https://doi.org/10.1007/s11626-022-00736-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11626-022-00736-0