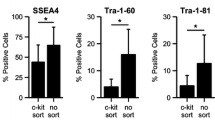
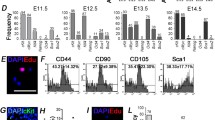
Abstract
Amniotic fluid (AF) is a rich source of mesenchymal stromal cells (MSCs) that have the ability to differentiate into multiple lineages rendering them a promising and powerful tool for regenerative medicine. However, information regarding the differences among AFMSCs derived from different gestational stages is limited. In the present study, AFMSCs derived from 125 pregnant rats at four embryonic day (E) stages (E12, E15, E18, and E21) were isolated and cultured. The primary E15 cells were the smallest in size and the easiest to culture and usually grew in a spherical shape that resembled the growth morphology of embryonic stem cells (ESCs). Once adhered, the E12 and E15 AFMSCs grew faster and could be passaged more than 60 times while still maintaining a continuous proliferative state; however, AFMSCs derived from E18 and E21 could normally be maintained for only 10 passages. To identify the possible reasons for this difference, RT-qPCR was used to examine several genes associated with self-renewal ability and cell origin. The Sox2 expression levels indicated that AFMSCs from E12 and E15 possessed stronger self-renewal capability. The K19, Col2A1, FGF5, AFP, and SPC expression levels indicated there were mixed-population cells co-existing in the AFMSC culture. In conclusion, E15 cells were easier to culture than E12 cells, could be passaged more often, and had a higher Sox2 expression than E18 or E21 cells. The E15-derived AFMSCs had higher viability and proliferative capacity than cells from the later stages. Therefore, AF cells from the early stages could be a good choice for exploring potential treatments involving AFMSCs.







Similar content being viewed by others
References
Akagi T, Kuure S, Uranishi K, Koide H, Costantini F, Yokota T (2015) ETS-related transcription factors ETV4 and ETV5 are involved in proliferation and induction of differentiation-associated genes in embryonic stem (ES) cells. J Biol Chem 290:22460–22473
Alessio N, Pipino C, Mandatori D, Di Tomo P, Ferone A, Marchiso M, Melone MAB, Peluso G, Pandolfi A, Galderisi U (2018) Mesenchymal stromal cells from amniotic fluid are less prone to senescence compared to those obtained from bone marrow: an in vitro study. J Cell Physiol 233:8996–9006
Billing AM, Ben Hamidane H, Dib SS, Cotton RJ, Bhagwat AM, Kumar P, Hayat S, Yousri NA, Goswami N, Suhre K, Rafii A, Graumann J (2016) Comprehensive transcriptomic and proteomic characterization of human mesenchymal stem cells reveals source specific cellular markers. Sci Rep 6:21507
Caplan AI (1991) Mesenchymal stem cells. J Orthop Res 9:641–650
Cavaleri F, Scholer HR (2003) Nanog: a new recruit to the embryonic stem cell orchestra. Cell 113:551–557
Chen W, Siegel N, Li L, Pollak A, Hengstschläger M, Lubec G (2009) Variations of protein levels in human amniotic fluid stem cells CD117/2 over passages 5-25. J Proteome Res 8:5285–5295
De Coppi P, Bartsch G Jr, Siddiqui MM, Xu T, Santos CC, Perin L, Mostoslavsky G, Serre AC, Snyder EY, Yoo JJ, Furth ME, Soker S, Atala A (2007) Isolation of amniotic stem cell lines with potential for therapy. Nat Biotechnol 25:100–106
Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop D, Horwitz E (2006) Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 8:315–317
Dostert G, Mesure B, Menu P, Velot E (2017) How do mesenchymal stem cells influence or are influenced by microenvironment through extracellular vesicles communication? Front Cell Dev Biol 5:6
Esteves CL, Sheldrake TA, Dawson L, Menghini T, Rink BE, Amilon K, Khan N, Péault B, Donadeu FX (2017) Equine mesenchymal stromal cells retain a pericyte-like phenotype. Stem Cells Dev 26:964–972
Fernandes RA, Wenceslau CV, Reginato AL, Kerkis I, Miglino MA (2012) Derivation and characterization of progenitor stem cells from canine allantois and amniotic fluids at the third trimester of gestation. Placenta. 33:640–644
Friedenstein AJ (1976) Precursor cells of mechanocytes. Int Rev Cytol 47:327–359
Galipeau J, Sensébé L (2018) Mesenchymal stromal cells: clinical challenges and therapeutic opportunities. Cell Stem Cell 22:824–833
Harrell CR, Gazdic M, Fellabaum C, Jovicic N, Djonov V, Arsenijevic N, Volarevic V (2019) Therapeutic potential of amniotic fluid derived mesenchymal stem cells based on their differentiation capacity and immunomodulatory properties. Curr Stem Cell Res Ther 14:327–336
Hill MA (2019) Embryology rat development. https://embryology.med.unsw.edu.au/embryology/index.php/Rat_Development. Accessed 19 Jan 2019
Hoepfner J, Kleinsorge M, Papp O, Ackermann M, Alfken S, Rinas U, Solodenko W, Kirschning A, Sgodda M, Cantz T (2016) Biphasic modulation of Wnt signaling supports efficient foregut endoderm formation from human pluripotent stem cells. Cell Biol Int 40:534–548
Honarpardaz A, Irani S, Pezeshki-Modaress M, Zandi M, Sadeghi A (2019) Enhanced chondrogenic differentiation of bone marrow mesenchymal stem cells on gelatin/glycosaminoglycan electrospun nanofibers with different amount of glycosaminoglycan. J Biomed Mater Res A 107:38–48
Irfan-Maqsood M, Matin MM, Heirani-Tabasi A, Bahrami M, Naderi-Meshkin H, Mirahmadi M, Hassanzadeh H, Sanjar Moussavi N, Raza-Shah H, Raeesolmohaddeseen M, Bidkhori H, B. AR. (2016) Adipose derived mesenchymal stem cells express keratinocyte lineage markers in a co-culture model. Cell Mol Biol (Noisy-le-grand) 62:44–54
Jensen TJ, Shui JE, Finck CM (2017) The effect of meconium exposure on the expression and differentiation of amniotic fluid mesenchymal stem cells. J Neonatal-Perinatal Med 10:313–323
Kaviani A, Perry TE, Dzakovic A, Jennings RW, Ziegler MM, Fauza DO (2001) The amniotic fluid as a source of cells for fetal tissue engineering. J Pediatr Surg 36:1662–1665
Lesage F, Pranpanus S, Bosisio FM, Jacobs M, Ospitalieri S, Toelen J, Deprest J (2017) Minimal modulation of the host immune response to SIS matrix implants by mesenchymal stem cells from the amniotic fluid. Hernia. 21:973–982
Li Y, Gu C, Xu W, Yan J, Xia Y, Ma Y, Chen C, He X, Tao H (2014) Therapeutic effects of amniotic fluid-derived mesenchymal stromal cells on lung injury in rats with emphysema. Respir Res 15:120
Liu TM, Lee EH, Lim B, Shyh-Chang N (2016) Concise review: balancing stem cell self-renewal and differentiation with PLZF. Stem Cells 34:277–287
Moschidou D, Mukherjee S, Blundell MP, Drews K, Jones GN, Abdulrazzak H, Nowakowska B, Phoolchund A, Lay K, Ramasamy TS, Cananzi M, Nettersheim D, Sullivan M, Frost J, Moore G, Vermeesch JR, Fisk NM, Thrasher AJ, Atala A, Adjaye J, Schorle H, De Coppi P, Guillot PV (2012) Valproic acid confers functional pluripotency to human amniotic fluid stem cells in a transgene-free approach. Mol Ther 20:1953–1967
Mun-Fun H, Ferdaos N, Hamzah SN, Ridzuan N, Hisham NA, Abdullah S, Ramasamy R, Cheah PS, Thilakavathy K, Yazid MN, Nordin N (2015) Rat full term amniotic fluid harbors highly potent stem cells. Res Vet Sci 102:89–99
Pines M, Hurwitz S (1991) The role of the growth plate in longitudinal bone growth. Poult Sci 70:1806–1814
Pratheesh MD, Gade NE, Katiyar AN, Dubey PK, Sharma B, Saikumar G, Amarpal, Sharma GT (2013) Isolation, culture and characterization of caprine mesenchymal stem cells derived from amniotic fluid. Res Vet Sci 94:313–319
Rahman MS, Spitzhorn LS, Wruck W, Hagenbeck C, Balan P, Graffmann N, Bohndorf M, Ncube A, Guillot PV, Fehm T, Adjaye J (2018) The presence of human mesenchymal stem cells of renal origin in amniotic fluid increases with gestational time. Stem Cell Res Ther 9:113
Rodda DJ, Chew JL, Lim LH, Loh YH, Wang B, Ng HH, Robson P (2005) Transcriptional regulation of nanog by OCT4 and SOX2. J Biol Chem 280:24731–24737
Rossi B, Merlo B, Colleoni S, Iacono E, Tazzari PL, Ricci F, Lazzari G, Galli C (2014) Isolation and in vitro characterization of bovine amniotic fluid derived stem cells at different trimesters of pregnancy. Stem Cell Rev 10:712–724
Roubelakis MG, Bitsika V, Zagoura D, Trohatou O, Pappa KI, Makridakis M, Antsaklis A, Vlahou A, Anagnou NP (2011) In vitro and in vivo properties of distinct populations of amniotic fluid mesenchymal progenitor cells. J Cell Mol Med 15:1896–1913
Samsonraj RM, Rai B, Sathiyanathan P, Puan KJ, Rötzschke O, Hui JH, Raghunath M, Stanton LW, Nurcombe V, Cool SM (2015) Establishing criteria for human mesenchymal stem cell potency. Stem Cells 33:1878–1891
Savickiene J, Treigyte G, Baronaite S, Valiuliene G, Kaupinis A, Valius M, Arlauskiene A, Navakauskiene R (2015) Human amniotic fluid mesenchymal stem cells from second- and third-trimester amniocentesis: differentiation potential, molecular signature, and proteome Analysis. Stem Cells Int 2015:1–15
Savickienė J, Baronaitė S, Zentelytė A, Treigytė G, Navakauskienė R (2016) Senescence-associated molecular and epigenetic alterations in mesenchymal stem cell cultures from amniotic fluid of normal and fetus-affected pregnancy. Stem Cells Int 2016:1–13
Schiavo AA, Franzin C, Albiero M, Piccoli M, Spiro G, Bertin E, Urbani L, Visentin S, Cosmi E, Fadini GP, De Coppi P, Pozzobon M (2015) Endothelial properties of third-trimester amniotic fluid stem cells cultured in hypoxia. Stem Cells Ther 6:209
Spitzhorn LS, Rahman MS, Schwindt L, Ho HT, Wruck W, Bohndorf M, Wehrmeyer S, Ncube A, Beyer I, Hagenbeck C, Balan P, Fehm T, Adjaye J (2017) Isolation and molecular characterization of amniotic fluid-derived mesenchymal stem cells obtained from caesarean sections. Stem Cells Int 2017:5932706
Underwood MA, Gilbert WM, Sherman MP (2005) Amniotic fluid: not just fetal urine anymore. J Perinatol 25:341–348
Zheng Y, Gao Z, Xie C, Zhu H, Peng L, Chen J, Chong Y (2008) Characterization and hepatogenic differentiation of mesenchymal stem cells from human amniotic fluid and human bone marrow: a comparative study. Cell Biol Int 32:1439–1448
Funding
This work was supported by the National Natural Science Foundation of China (Grant numbers: 81871219, 81671469, 81901565) and the National Key Research and Development Program (2016YFC1000505).
Author information
Authors and Affiliations
Corresponding author
Additional information
Editor: Tetsuji Okamoto
Rights and permissions
About this article
Cite this article
Huang, J., Ma, W., Wei, X. et al. Amniotic fluid mesenchymal stromal cells from early stages of embryonic development have higher self-renewal potential. In Vitro Cell.Dev.Biol.-Animal 56, 701–714 (2020). https://doi.org/10.1007/s11626-020-00511-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11626-020-00511-z