Abstract
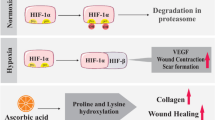
The differences between oral mucosa and skin wound healing involving hypoxic responses of fibroblasts are poorly elucidated. In this study, we aimed to study the different hypoxic responses between oral and skin fibroblasts embedded in a three-dimensional (3D) collagen matrix to address the early stage of wound healing. Primary oral mucosa fibroblasts (OMFs) obtained from the retromolar area and skin fibroblasts (SFs) obtained from the abdomen were cultured in the 3D ‘floating model’ under either 21%, 5% or 1% O2 for 2 days. Cell viability under hypoxia was higher in the OMFs than in the SFs. Collagen gel contraction was suppressed under hypoxic conditions in both fibroblasts, consistent with the reduction of alpha smooth muscle actin expression, except for SFs under 1% O2. Subsequently, their gene expression profiles between 21 and 1% O2 concentrations were compared via microarray technology, and the expression profiles of the extracellular matrix (ECM)-associated proteins, including matrix metalloproteinases and collagens, were evaluated. The OMFs were more susceptible to 1% O2, and more of their genes were downregulated than the SFs’. Although the production and expression levels of ECM-associated proteins in both fibroblasts diminished under hypoxia, those levels in OMFs were significantly higher than those in SFs. In the case of single origin OMFs and SFs, our findings suggest that OMFs possess a higher baseline production capacity of several ECM-associated proteins than SFs, except type III collagen. The intrinsic hypoxic responses of OMFs may be attributed to a more favourable wound healing in oral mucosa.








Similar content being viewed by others
References
Bochaton-Piallat ML, Gabbiani G, Hinz B (2016) The myofibroblast in wound healing and fibrosis: answered and unanswered questions. F1000Res 5:752. https://doi.org/10.12688/f1000research.8190.1
Brown RA (2013) In the beginning there were soft collagen-cell gels: towards better 3D connective tissue models? Exp Cell Res 319(16):2460–2469. https://doi.org/10.1016/j.yexcr.2013.07.001
Brown RA, Phillips JB (2007) Cell responses to biomimetic protein scaffolds used in tissue repair and engineering. Int Rev Cytol 262:75–150
Caley MP, Martins VLC, O’Toole EA (2015) Metalloproteinases and wound healing. Adv Wound Care (New Rochelle) 4(4):225–234. https://doi.org/10.1089/wound.2014.0581
Carlson MA, Smith LM, Cordes CM, Chao J, Eudy JD (2013) Attachment-regulated signalling networks in the fibroblast-populated 3D collagen matrix. Sci Rep 3:1880. https://doi.org/10.1038/srep01880
Chen L, Arbieva ZH, Guo S, Marucha PT, Mustoe TA, DiPietro LA (2010) Positional differences in the wound transcriptome of skin and oral mucosa. BMC Genomics 11:471. https://doi.org/10.1186/1471-2164-11-471
Chen L, Gajendrareddy PK, DiPietro LA (2012) Differential expression of HIF-1α in skin and mucosal wounds. J Dent Res 91(9):871–876. https://doi.org/10.1177/0022034512454435
Chiquet M, Katsaros C, Kletsas D (2000) (2015) multiple functions of gingival and mucoperiosteal fibroblasts in oral wound healing and repair. Periodontol 68(1):21–40. https://doi.org/10.1111/prd.12076
Ebisawa K, Kato R, Okada M, Sugimura T, Latif MA, Hori Y, Narita Y, Ueda M, Honda H, Kagami H (2011) Gingival and dermal fibroblasts: their similarities and differences revealed from gene expression. J Biosci Bioeng 111(3):255–258
Enoch S, Peake MA, Wall I, Davies L, Farrier J, Giles P, Kipling D, Price P, Moseley R, Thomas D, Stephens P (2010) ‘Young’ oral fibroblasts are geno/phenotypically distinct. Dent Res 89(12):1407–1413. https://doi.org/10.1177/0022034510377796
Evensen NA, Li Y, Kuscu C, Liu J, Cathcart J, Banach A, Zhang Q, Li E, Joshi S, Yang J, Denoya PI, Pastorekova S, Zucker S, Shroyer KR, Cao J (2015) Hypoxia promotes colon cancer dissemination through up-regulation of cell migration-inducing protein (CEMIP). Oncotarget 6(24):20723–20739
Fan Z, Xu Z, Niu H, Gao N, Guan Y, Li C, Dang Y, Cui X, Liu XL, Duan Y, Li H, Zhou X, Lin PH, Ma J, Guan J (2018) An injectable oxygen release system to augment cell survival and promote cardiac repair following myocardial infarction. Sci Rep 8:1371. https://doi.org/10.1038/s41598-018-19906-w
Faulknor RA, Olekson MA, Nativ NI, Ghodbane M, Gray AJ1, Berthiaume F (2015) Mesenchymal stromal cells reverse hypoxia-mediated suppression of α-smooth muscle actin expression in human dermal fibroblasts. Biochem Biophys Res Commun. 458(1):8–13. https://doi.org/10.1016/j.bbrc.2015.01.013.
Glim JE, van Egmond M, Niessen FB, Everts V, Beelen RH (2013) Detrimental dermal wound healing: what can we learn from the oral mucosa? Wound Repair Regen 21(5):648–660. https://doi.org/10.1111/wrr.12072
Green JA, Yamada KM (2007) Three-dimensional microenvironments modulate fibroblast signaling responses. Adv Drug Deliv Rev 59(13):1293–1298
Grinnell F (2003) Fibroblast biology in three-dimensional collagen matrices. Trends Cell Biol 13(5):264–269
Grinnell F, Petroll WM (2010) Cell motility and mechanics in three-dimensional collagen matrices. Annu Rev Cell Dev Biol 26:335–361. https://doi.org/10.1146/annurev.cellbio.042308.113318
Häkkinen L, Larjava H, Koivisto L (2012) Granulation tissue formation and remodeling. Endod Top 24:94–129. https://doi.org/10.1111/etp.12008
Hiraoka C, Toki F, Shiraishi K, Sayama K, Nishimura EK, Miura H, Higashiyama S, Nanba D (2016) Two clonal types of human skin fibroblasts with different potentials for proliferation and tissue remodeling ability. J Dermatol Sci 82(2):84–94. https://doi.org/10.1016/j.jdermsci.2016.01.009
Hong WX, Hu MS, Esquivel M, Liang GY, Rennert RC, McArdle A, Paik KJ, Duscher D, Gurtner GC, Lorenz HP, Longaker MT (2014) The role of hypoxia-inducible factor in wound healing. Adv Wound Care (New Rochelle) 3(5):390–399
Itaya T, Hirai T, Hirai T, Numoto H, Takeda H, Ueda M (2017) The use of fibroblasts for ameliorating structural changes associated with skin aging. Rejuvenation Res 20(5):383–388. https://doi.org/10.1089/rej.2016.1902
Johnson A, Francis M, DiPietro LA (2014) Differential apoptosis in mucosal and dermal wound healing. Adv Wound Care (New Rochelle) 3(12):751–761. https://doi.org/10.1089/wound.2012.0418
Kang S, Lee D, Theusch BE, Arpey CJ, Brennan TJ (2013) Wound hypoxia in deep tissue after incision in rats. Wound Repair Regen 21(5):730–739. https://doi.org/10.1111/wrr.12081
Kanta J (2015) Collagen matrix as a tool in studying fibroblastic cell behavior. Cell Adhes Migr 9(4):308–316. https://doi.org/10.1080/19336918.2015.1005469
Larjava H, Wiebe C, Gallant-Behm C, Hart DA, Heino J, Häkkinen L (2011) Exploring scarless healing of oral soft tissues. J Can Dent Assoc 77:b18
Leinhos L, Peters J, Krull S, Helbig L, Vogler M, Levay M, van Belle GJ, Ridley AJ, Lutz S, Katschinski DM, Zieseniss A (2019) Hypoxia suppresses myofibroblast differentiation by changing RhoA activity. J Cell Sci 18:132(5). https://doi.org/10.1242/jcs.223230
Li L, Yan LH, Manoj S, Li Y, Lu L (2017) Central role of CEMIP in tumorigenesis and its potential as therapeutic target. J Cancer 8(12):2238–2246. https://doi.org/10.7150/jca.19295
Lutolf MP, Hubbell JA (2005) Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat Biotechnol 23(1):47–55
Lynam EC, Xie Y, Dawson R, Mcgovern J, Upton Z, Wang X (2015) Severe hypoxia and malnutrition collectively contribute to scar fibroblast inhibition and cell apoptosis. Wound Repair Regen 23(5):664–671. https://doi.org/10.1111/wrr.12343
Lynch MD, Watt FM (2018) Fibroblast heterogeneity: implications for human disease. J Clin Invest 128(1):26–35. https://doi.org/10.1172/JCI93555
Ma L, Zhang B, Zhou C, Li Y, Li B, Yu M, Luo Y, Gao L, Zhang D, Xue Q, Qiu Q, Lin B, Zou J, Yang H (2018) The comparison genomics analysis with glioblastoma multiforme (GBM) cells under 3D and 2D cell culture conditions. Colloids Surf B: Biointerfaces 172:665–673. https://doi.org/10.1016/j.colsurfb.2018.09.034
Mah W, Jiang G, Olver D, Cheung G, Kim B, Larjava H, Häkkinen L (2014) Human gingival fibroblasts display a non-fibrotic phenotype distinct from skin fibroblasts in three-dimensional cultures. PLoS One 9(3):e90715. https://doi.org/10.1371/journal.pone.0090715
McKeown ST, Barnes JJ, Hyland PL, Lundy FT, Fray MJ, Irwin CR (2007) Matrix metalloproteinase-3 differences in oral and skin fibroblasts. J Dent Res 86(5):457–462
Modarressi A, Pietramaggiori G, Godbout C, Vigato E, Pittet B, Hinz B (2010) Hypoxia impairs skin myofibroblast differentiation and function. J Invest Dermatol 130(12):2818–2827. https://doi.org/10.1038/jid.2010.224
Mohd Nor NH, Berahim Z, Azlina A, Mokhtar KI, Kannan TP (2017) Identification and characterization of intraoral and dermal fibroblasts revisited. Curr Stem Cell Res Ther 12(8):675–681. https://doi.org/10.2174/1574888X12666170929124621
Moore AL, Marshall CD, Barnes LA, Murphy MP, Ransom RC, Longaker MT (2018) Scarless wound healing: transitioning from fetal research to regenerative healing. Wiley Interdiscip Rev Dev Biol 7(2):e309. https://doi.org/10.1002/wdev.309
Müller AS, Janjić K, Lilaj B, Edelmayer M, Agis H (2017) Hypoxia-based strategies for regenerative dentistry-views from the different dental fields. Arch Oral Biol 81:121–130. https://doi.org/10.1016/j.archoralbio.2017.04.029
Rechardt O, Elomaa O, Vaalamo M, Pääkkönen K, Jahkola T, Höök-Nikanne J, Hembry RM, Häkkinen L, Kere J, Saarialho-Kere U (2000) Stromelysin-2 is upregulated during normal wound repair and is induced by cytokines. J Invest Dermatol 115(5):778–787
Ruthenborg RJ, Ban JJ, Wazir A, Takeda N, Kim JW (2014) Regulation of wound healing and fibrosis by hypoxia and hypoxia-inducible factor-1. Mol Cell 37(9):637–643. https://doi.org/10.14348/molcells.2014.0150
Sen CK, Khanna S, Roy S (2006) Perceived hyperoxia: oxygen-induced remodeling of the reoxygenated heart. Cardiovasc Res 71(2):280–288
Shannon DB, McKeown ST, Lundy FT, Irwin CR (2006) Phenotypic differences between oral and skin fibroblasts in wound contraction and growth factor expression. Wound Repair Regen 14(2):172–178
Sriram G, Bigliardi PL, Bigliardi-Qi M (2015) Fibroblast heterogeneity and its implications for engineering organotypic skin models in vitro. Eur J Cell Biol 94(11):483–512. https://doi.org/10.1016/j.ejcb.2015.08.001
Steinbrech DS, Longaker MT, Mehrara BJ, Saadeh PB, Chin GS, Gerrets RP, Chau DC, Rowe NM, Gittes GK (1999) Fibroblast response to hypoxia: the relationship between angiogenesis and matrix regulation. J Surg Res 84(2):127–133
Stephanie RG, Diegelmann RF (2006) Basic science of wound healing. Critical Limb Ischemia:131–136
Szpaderska AM, Zuckerman JD, DiPietro LA (2003) Differential injury responses in oral mucosal and cutaneous wounds. J Dent Res 82(8):621–626. https://doi.org/10.1016/j.jbiosc.2010.11.014
Tandara AA, Mustoe TA (2004) Oxygen in wound healing--more than a nutrient. World J Surg 28(3):294–300
Toki F, Honkura N, Shirakata Y, Imamura T, Higashiyama S, Nanba D (2013) Second harmonic generation reveals collagen fibril remodeling in fibroblast-populated collagen gels. Cell Struct Funct 38(2):227–236. https://doi.org/10.1016/j.jphotochemrev.2017.01.001
Uenoyama A, Kakizaki I, Shiomi A, Saito N, Hara Y, Saito T, Ohnuki H, Kato H, Takagi R, Maeda T, Izumi K (2016) Effects of C-xylopyranoside derivative on epithelial regeneration in an in vitro 3D oral mucosa model. Biosci Biotechnol Biochem 80(7):1344–1355. https://doi.org/10.1080/09168451.2016.1153957
Woodley DT (2017) Distinct fibroblasts in the papillary and reticular dermis: implications for wound healing. Dermatol Clin 35(1):95–100. https://doi.org/10.1016/j.det.2016.07.004
Yoshida H, Nagaoka A, Komiya A, Aoki M, Nakamura S, Morikawa T, Ohtsuki R, Sayo T, Okada Y, Takahashi Y (2018) Reduction of hyaluronan and increased expression of HYBID (alias CEMIP and KIAA1199) correlate with clinical symptoms in photoaged skin. Br J Dermatol 179(1):136–144. https://doi.org/10.1111/bjd.16335
Yoshida H, Nagaoka A, Kusaka-Kikushima A, Tobiishi M, Kawabata K, Sayo T, Sakai S, Sugiyama Y, Enomoto H, Okada Y, Inoue S (2013) KIAA1199, a deafness gene of unknown function, is a new hyaluronan binding protein involved in hyaluronan depolymerization. Proc Natl Acad Sci U S A 110(14):5612–5617. https://doi.org/10.1073/pnas.1215432110
Yoshihara T, Hirakawa Y, Hosaka M, Nangaku M, Tobita S (2017) Oxygen imaging of living cells and tissues using luminescent molecular probes. J Photochem Photobiol C: Photochem Rev 30:71–95. https://doi.org/10.1016/j.archoralbio.2018.02.008
Zhang S, Buttler-Buecher P, Denecke B, Arana-Chavez VE, Apel C (2018) A comprehensive analysis of human dental pulp cell spheroids in a three-dimensional pellet culture system. Arch Oral Biol 91:1–8. https://doi.org/10.1016/j.archoralbio.2018.02.008
Zhang Y, Jia S, Jiang WG (2014) KIAA1199 and its biological role in human cancer and cancer cells (review). Oncol Rep 31(4):1503–1508. https://doi.org/10.3892/or.2014.3038
Author contributions
Conceived and designed the work: YH-S KI. Performed the experiments: YH-S KH NS AS AU RT. Analysed the data: YH-S KH KI. Contributed reagents/materials/analysis tools: YH-S KH NS AS AU RT. Visualised the data: YH-S KH KI. Wrote the original paper: YH-S KI. Reviewed and edited the paper: YH-S RT KI.
Funding
This research was supported in part by both JSPS KAKENHI Grant Numbers 18H06290G to YH-S and 17H04398G to KI.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Statement of ethics
The use of human oral mucosa fibroblasts and the procurement procedure was approved by the Internal Review Board of the Niigata University Hospital. Number: 2015-5018.
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Editor: Tetsuji Okamoto
Appendices
Appendix 1
A list of 207 and 560 upregulated and downregulated genes in OMFs, a list of 62 and 43 upregulated and downregulated genes in SFs and a list of 62 and 47 upregulated and downregulated genes in both OMFs and SFs, at 1% O2 concentration, respectively.
Appendix 2
Gene annotation enrichment analysis of hypoxia-upregulated genes in OMFs cultured in the ‘floating model’ was performed using DAVID Bioinformatics Resources 6.8. The biological process terms were extracted when the p-value was <0.01. The asterisks indicate the GO terms related to ECM remodelling, hypoxic response and cell viability. The annotation gene lists of these GO terms are shown in the following Appendix 6.
Appendix 3
Gene annotation enrichment analysis of hypoxia-downregulated genes in OMFs cultured in the ‘floating model’ was performed using DAVID Bioinformatics Resources 6.8. The biological process terms were extracted when the p-value was <0.01. The asterisks indicate the GO terms related to ECM remodelling, hypoxic response and cell viability. The annotation gene lists of these GO terms are shown in the following Appendix 7.
Appendix 4
Gene annotation enrichment analysis of hypoxia-upregulated genes in SFs cultured in the ‘floating model’ was performed using DAVID Bioinformatics Resources 6.8. The biological process terms were extracted when the p-value was <0.01. The asterisks indicate the GO terms related to ECM remodelling, hypoxic response and cell viability. The annotation gene lists of these GO terms are shown in the following Appendix 8.
Appendix 5
Gene annotation enrichment analysis of hypoxia-downregulated genes in SFs cultured in the ‘floating model’ was performed using DAVID Bioinformatics Resources 6.8. The biological process terms were extracted when the p-value was <0.01. The asterisks indicate the GO terms related to ECM remodelling, hypoxic response and cell viability. The annotation gene lists of these GO terms are shown in the following Appendix 9.
Appendix 6
Based on a functional annotation chart of hypoxia-upregulated genes in OMFs cultured in the ‘floating model’, genes related to ECM remodelling, hypoxic response and cell viability (indicated by eight asterisks in Appendix 2) were listed.
Appendix 7
Based on a functional annotation chart of hypoxia-downregulated genes in OMFs cultured in the ‘floating model’, genes related to ECM remodelling, hypoxic response and cell viability (indicated by five asterisks in Appendix 3) were listed.
Appendix 8
Based on a functional annotation chart of hypoxia-upregulated genes in SFs cultured in the ‘floating model’, genes related to hypoxic response (indicated by two asterisks in Appendix 4) were listed.
Appendix 9
Based on a functional annotation chart of hypoxia-downregulated genes in SFs cultured in the ‘floating model’, genes related to ECM remodelling and cell viability (indicated by two asterisks in Appendix 5) were listed.
Rights and permissions
About this article
Cite this article
Hara-Saito, Y., Kato, H., Saito, N. et al. Distinct differences in hypoxic responses between human oral mucosa and skin fibroblasts in a 3D collagen matrix. In Vitro Cell.Dev.Biol.-Animal 56, 452–479 (2020). https://doi.org/10.1007/s11626-020-00458-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11626-020-00458-1