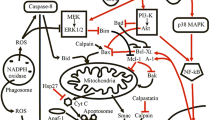
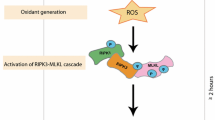
Abstract
Neutrophils undergo cell death processes once their physiological function has been fulfilled. Apoptosis, necrosis, pyroptosis, or NETosis, a unique form of cell death, could occur, depending on the type of stimulant or inhibitory intervention. We investigated whether phorbol myristate acetate (PMA) and Klebsiella pneumoniae (KP), serving as stimulants, or whether an inhibitor (cytochalasin B, CytB) could alter the morphology and gene expression pattern associated with mouse neutrophil cell death. Fluorescence microscopy, flow cytometry, and real-time PCR approaches were used to identify morphological changes, percentages of cell death, and gene expression patterns, respectively. The results showed an increase in the percentage of cell death in PMA and KP groups, whereas CytB exerted inhibitory effects. Fluorescently stained cell nuclei revealed significantly different percentages of cell death among treatments. Moreover, there was a stepwise increase in cell death from 90 to 150 min after stimulation. Quantification of the release of neutrophil extracellular traps (NETs) demonstrated clearly elevated amounts in cells stimulated with KP, while the amount of NETs was slightly increased or unchanged with PMA or CytB treatments. Analysis of the genes involved in cell death revealed that mRNA expression of CASP1, IL1β, IL18, MCL1, NLRP3, and PYCARD was down-regulated in the PMA group, with the exception of AIM2 and CASP3. The expression of AIM2, IL1β, MCL1, and NLRP3 was up-regulated in KP-stimulated neutrophils, while CASP1, CASP3, IL18, and PYCARD genes were down-regulated. In summary, a spectrum of specific cell stimulants and exposure durations accounted for different outcomes in mouse neutrophil cell death.






Similar content being viewed by others
References
Abe J, Morrell C (2016) Pyroptosis as a regulated form of necrosis: PI+/annexin V−/high caspase 1/low caspase 9 activity in cells = pyroptosis? Circ Res 118:1457–1460
Bachoual R, Talmoudi W, Boussetta T, Braut F, El-Benna J (2011) An aqueous pomegranate peel extract inhibits neutrophil myeloperoxidase in vitro and attenuates lung inflammation in mice. Food Chem Toxicol 49:1224–1228
Boucher D, Chen KW, Schroder K (2015) Burn the house, save the day: pyroptosis in pathogen restriction. Inflammasome 2:1–6
Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, Weinrauch Y, Zychlinsky A (2004) Neutrophil extracellular traps kill bacteria. Science 303:1532–1535
Brinkmann V, Zychlinsky A (2007) Beneficial suicide: why neutrophils die to make NETs. Nat Rev Microbiol 5:577–582
Brinkmann V, Zychlinsky A (2012) Neutrophil extracellular traps: is immunity the second function of chromatin? J Cell Biol 198:773–783
Bruns S, Kniemeyer O, Hasenberg M, Aimanianda V, Nietzsche S, Thywißen A, Jeron A, Latgé J-P, Brakhage AA, Gunzer M (2010) Production of extracellular traps against Aspergillus fumigatus in vitro and in infected lung tissue is dependent on invading neutrophils and influenced by hydrophobin RodA. PLoS Pathog 6:e1000873
Cai S, Batra S, Del Piero F, Jeyaseelan S (2016) NLRP12 modulates host defense through IL-17A–CXCL1 axis. Mucosal Immunol 9:503–514
Chen KW, Groß CJ, Sotomayor FV, Stacey KJ, Tschopp J, Sweet MJ, Schroder K (2014) The neutrophil NLRC4 inflammasome selectively promotes IL-1β maturation without pyroptosis during acute Salmonella challenge. Cell Rep 8:570–582
Desai J, Kumar SV, Mulay SR, Konrad L, Romoli S, Schauer C, Herrmann M, Bilyy R, Müller S, Popper B, Nakazawa D, Weidenbusch M, Thomasova D, Krautwald S, Linkermann A, Anders HJ (2016) PMA and crystal-induced neutrophil extracellular trap formation involves RIPK1-RIPK3-MLKL signaling. Eur J Immunol 46:223–229
Eisen MB, Spellman PT, Brown PO, Botstein D (1998) Cluster analysis and display of genome-wide expression patterns. Proc Natl Acad Sci U S A 95:14863–14868
Ermert D, Urban CF, Laube B, Goosmann C, Zychlinsky A, Brinkmann V (2009) Mouse neutrophil extracellular traps in microbial infections. J Innate Immun 1:181–193
Farrera C, Fadeel B (2013) Macrophage clearance of neutrophil extracellular traps is a silent process. J Immunol 191:2647–2656
Fernandes-Alnemri T, Wu J, Yu JW, Datta P, Miller B, Jankowski W, Rosenberg S, Zhang J, Alnemri ES (2007) The pyroptosome: a supramolecular assembly of ASC dimers mediating inflammatory cell death via caspase-1 activation. Cell Death Differ 14:1590–1604
Fitzgerald KA (2010) NLR-containing inflammasomes: central mediators of host defense and inflammation. Eur J Immunol 40:595–598
Fox S, Leitch AE, Duffin R, Haslett C, Rossi AG (2010) Neutrophil apoptosis: relevance to the innate immune response and inflammatory disease. J Innate Immun 2:216–227
Galluzzi L, Vitale I, Abrams J, Alnemri ES, Baehrecke E, Blagosklonny M, Dawson T, Dawson V, El-Deiry W, Fulda S, Gottlieb E, Green DR, Hengartner MO, Kepp O, Knight RA, Kumar S, Lipton SA, Lu X, Madeo F, Malorni W, Mehlen P, Nuñez G, Peter ME, Piacentini M, Rubinsztein DC, Shi Y, Simon HU, Vandenabeele P, White E, Yuan J, Zhivotovsky B, Melino G, Kroemer G (2012) Molecular definitions of cell death subroutines: recommendations of the Nomenclature Committee on Cell Death 2012. Cell Death Differ 19:107–120
Gargiulo S, Greco A, Gramanzini M, Esposito S, Affuso A, Brunetti A, Vesce G (2012) Mice anesthesia, analgesia, and care, part I: anesthetic considerations in preclinical research. ILAR J 53:E55–E69
Geering B, Simon HU (2011) Peculiarities of cell death mechanisms in neutrophils. Cell Death Differ 18:1457–1469
Gupta AK, Hasler P, Holzgreve W, Gebhardt S, Hahn S (2005) Induction of neutrophil extracellular DNA lattices by placental microparticles and IL-8 and their presence in preeclampsia. Hum Immunol 66:1146–1154
Henry CM, Hollville E, Martin SJ (2013) Measuring apoptosis by microscopy and flow cytometry. Methods 61:90–97
Iba T, Murai M, Nagaoka I, Tabe Y (2014) Neutrophil extracellular traps, damage-associated molecular patterns, and cell death during sepsis. Acute Med Surg 1:2–9
Jorgensen I, Miao EA (2015) Pyroptotic cell death defends against intracellular pathogens. Immunol Rev 265:130–142
Kahlenberg JM, Carmona-Rivera C, Smith CK, Kaplan MJ (2013) Neutrophil extracellular trap–associated protein activation of the NLRP3 inflammasome is enhanced in lupus macrophages. J Immunol 190:1217–1226
Karmakar M, Katsnelson M, Malak HA, Greene NG, Howell SJ, Hise AG, Camilli A, Kadioglu A, Dubyak GR, Pearlman E (2015) Neutrophil IL-1β processing induced by pneumolysin is mediated by the NLRP3/ASC inflammasome and caspase-1 activation and is dependent on K+ efflux. J Immunol 194:1763–1775
Karmakar M, Katsnelson MA, Dubyak GR, Pearlman E (2016) Neutrophil P2X7 receptors mediate NLRP3 inflammasome-dependent IL-1β secretion in response to ATP. Nat Commun 7:1–13
Kennedy AD, DeLeo FR (2009) Neutrophil apoptosis and the resolution of infection. Immunol Res 43:25–61
Khatua B, Bhattacharya K, Mandal C (2012) Sialoglycoproteins adsorbed by Pseudomonas aeruginosa facilitate their survival by impeding neutrophil extracellular trap through siglec-9. J Leukoc Biol 91:641–655
Kobayashi SD, Braughton KR, Palazzolo-Ballance AM, Kennedy AD, Sampaio E, Kristosturyan E, Whitney AR, Sturdevant DE, Dorward DW, Holland SM, Kreiswirth BN, Musser JM, DeLeo FR (2010) Rapid neutrophil destruction following phagocytosis of Staphylococcus aureus. J Innate Immun 2:560–575
Kobayashi SD, Porter AR, Dorward DW, Brinkworth AJ, Chen L, Kreiswirth BN, DeLeo FR (2016) Phagocytosis and killing of carbapenem-resistant ST258 Klebsiella pneumoniae by human neutrophils. J Infect Dis 213:1615–1622
Lim MBH, Kuiper JWP, Katchky A, Goldberg H, Glogauer M (2011) Rac2 is required for the formation of neutrophil extracellular traps. J Leukoc Biol 90:771–776
Liu J-J, Song C-W, Yue Y, Duan C-G, Yang J, He T, He Y-Z (2005) Quercetin inhibits LPS-induced delay in spontaneous apoptosis and activation of neutrophils. Inflamm Res 54:500–507
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔC T method. Methods 25:402–408
Luo Y, Dorf ME (2001) Isolation of mouse neutrophils. Curr Protoc Immunol 22:IV:3.20:3.20.1–3.20.6
Manzenreiter R, Kienberger F, Marcos V, Schilcher K, Krautgartner WD, Obermayer A, Huml M, Stoiber W, Hector A, Griese M, Hannig M, Studnicka M, Vitkov L, Hartl D (2012) Ultrastructural characterization of cystic fibrosis sputum using atomic force and scanning electron microscopy. J Cyst Fibros 11:84–92
Martinon F, Burns K, Tschopp J (2002) The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-β. Mol Cell 10:417–426
Medina E (2009) Neutrophil extracellular traps: a strategic tactic to defeat pathogens with potential consequences for the host. J Innate Immun 1:176–180
Miao EA, Leaf IA, Treuting PM, Mao DP, Dors M, Sarkar A, Warren SE, Wewers MD, Aderem A (2010) Caspase-1-induced pyroptosis is an innate immune effector mechanism against intracellular bacteria. Nat Immunol 11:1136–1142
Montojo J, Zuberi K, Rodriguez H, Kazi F, Wright G, Donaldson SL, Morris Q, Bader GD (2010) GeneMANIA Cytoscape plugin: fast gene function predictions on the desktop. Bioinformatics 26:2927–2928
Naccache PH, Fernandes MJ (2016) Challenges in the characterization of neutrophil extracellular traps: the truth is in the details. Eur J Immunol 46:52–55
National Research Council (2011) Guide for the care and use of laboratory animals, 8th edn. National Academies Press, Washington DC
Patankar YR, Mabaera R, Berwin B (2015) Differential ASC requirements reveal a key role for neutrophils and a noncanonical IL-1β response to Pseudomonas aeruginosa. Am J Physiol Lung Cell Mol Physiol 309:L902–L913
Pilsczek FH, Salina D, Poon KKH, Fahey C, Yipp BG, Sibley CD, Robbins SM, Green FHY, Surette MG, Sugai M, Bowden MG, Hussain M, Zhang K, Kubes P (2010) A novel mechanism of rapid nuclear neutrophil extracellular trap formation in response to Staphylococcus aureus. J Immunol 185:7413–7425
Remijsen Q, Vanden Berghe T, Wirawan E, Asselbergh B, Parthoens E, De Rycke R, Noppen S, Delforge M, Willems J, Vandenabeele P (2010) Neutrophil extracellular trap cell death requires both autophagy and superoxide generation. Cell Res 21:290–304
Sagulenko V, Thygesen SJ, Sester DP, Idris A, Cridland JA, Vajjhala PR, Roberts TL, Schroder K, Vince JE, Hill JM, Silke J, Stacey KJ (2013) AIM2 and NLRP3 inflammasomes activate both apoptotic and pyroptotic death pathways via ASC. Cell Death Differ 20:1149–1160
Sangaletti S, Tripodo C, Chiodoni C, Guarnotta C, Cappetti B, Casalini P, Piconese S, Parenza M, Guiducci C, Vitali C, Colombo MP (2012) Neutrophil extracellular traps mediate transfer of cytoplasmic neutrophil antigens to myeloid dendritic cells toward ANCA induction and associated autoimmunity. Blood 120:3007–3018
Simon H-U, Haj-Yehia A, Levi-Schaffer F (2000) Role of reactive oxygen species (ROS) in apoptosis induction. Apoptosis 5:415–418
Sturn A, Quackenbush J, Trajanoski Z (2002) Genesis: cluster analysis of microarray data. Bioinformatics 18:207–208
von Moltke J, Ayres JS, Kofoed EM, Chavarría-Smith J, Vance RE (2013) Recognition of bacteria by inflammasomes. Annu Rev Immunol 31:73–106
Wang X, Spandidos A, Wang H, Seed B (2012) PrimerBank: a PCR primer database for quantitative gene expression analysis, 2012 update. Nucleic Acids Res 40:D1144–D1149
Warde-Farley D, Donaldson SL, Comes O, Zuberi K, Badrawi R, Chao P, Franz M, Grouios C, Kazi F, Lopes CT, Maitland A, Mostafavi S, Montojo J, Shao Q, Wright G, Bader GD, Morris Q (2010) The GeneMANIA prediction server: biological network integration for gene prioritization and predicting gene function. Nucleic Acids Res 38:W214–W220
Watson RW, Redmond HP, Wang JH, Condron C, Bouchier-Hayes D (1996) Neutrophils undergo apoptosis following ingestion of Escherichia coli. J Immunol 156:3986–3992
Xi H, Zhang Y, Xu Y, Yang WY, Jiang X, Sha X, Cheng X, Wang J, Qin X, Yu J, Ji Y, Yang X, Wang H (2016) Caspase-1 inflammasome activation mediates homocysteine-induced pyrop-apoptosis in endothelial cells. Circ Res 118:1525–1539
Yan J, Meng X, Wancket LM, Lintner K, Nelin LD, Chen B, Francis KP, Smith CV, Rogers LK, Liu Y (2012) Glutathione reductase facilitates host defense by sustaining phagocytic oxidative burst and promoting the development of neutrophil extracellular traps. J Immunol 188:2316–2327
Ye P, Rodriguez FH, Kanaly S, Stocking KL, Schurr J, Schwarzenberger P, Oliver P, Huang W, Zhang P, Zhang J, Shellito JE, Bagby GJ, Nelson S, Charrier K, Peschon JJ, Kolls JK (2001) Requirement of interleukin 17 receptor signaling for lung CXC chemokine and granulocyte colony-stimulating factor expression, neutrophil recruitment, and host defense. J Exp Med 194:519–528
Zawrotniak M, Rapala-Kozik M (2013) Neutrophil extracellular traps (NETs) – formation and implications. Acta Biochim Pol 60:277–284
Acknowledgements
This study was supported by internal funding from the Faculty of Veterinary Medicine (Grant no. R000012003). We would like to thank the Medical Science Research Equipment Center for the use of a flow cytometer.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest regarding the publication of this paper.
Additional information
Editor: Tetsuji Okamoto
Nuttira Luehong and Juthamart Khaowmek contributed equally to this work.
Electronic supplementary material
Supplementary Fig. S1
(A) Representative peaks in melting profiles (dissociation curves) of the real-time PCR reactions. (B) Representative real-time PCR products of expressed genes in mouse bone marrow neutrophils on agarose gel electrophoresis and ethidium bromide staining. All PCR products had the correct size (see also Table 1 in “Materials and Methods” section). GAPDH was used as a reference gene. NTC = no template control, M = 25 bp molecular markers. (GIF 105 kb)
Rights and permissions
About this article
Cite this article
Luehong, N., Khaowmek, J., Wongsawan, K. et al. Preferential pattern of mouse neutrophil cell death in response to various stimulants. In Vitro Cell.Dev.Biol.-Animal 53, 513–524 (2017). https://doi.org/10.1007/s11626-016-0129-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11626-016-0129-7