Abstract
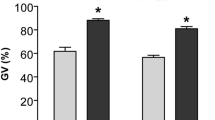
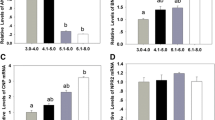
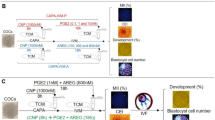
C-type natriuretic peptide (CNP) has been considered as a physiological meiotic inhibitor that stimulates the cGMP production by cumulus cell natriuretic peptide receptor 2 (NPR2), which inhibits oocyte phosphodiesterase type 3 activity and increases cAMP. In this study, we explored the effect of CNP pretreatment on the in vitro maturation (IVM) of bovine oocytes by examining changes in cleavage rate, blastocyst formation, mitochondrial DNA (mtDNA) copy number, reactive oxygen species (ROS) level, glutathione (GSH) content, and redox state. Our results showed that 200 nM CNP could effectively maintain meiotic arrest of bovine oocytes in vitro within 6 h. The two-step IVM system in which oocytes were pretreated with 200 nM CNP for 6 h and then cultured IVM for 28 h yielded a significantly (P < 0.05) increased blastocyst rate and cell number after in vitro fertilization (IVF) while compared to the conventional one-step IVM method. In addition, in comparison with the conventional 24-h matured oocyte, oocytes pretreated with 200 nM CNP for 6 h followed by 28 h IVM resulted in significantly (P < 0.05) higher mtDNA copy number and ROS levels in oocytes, while GSH level significantly (P < 0.05) decreased. Remarkably, regardless of treatment, no changes were observed in FAD++, NAD(P)H autofluorescence intensity, and redox ratio (FAD++/NAD(P)H) within the oocytes, maintaining a healthy metabolic equilibrium of redox throughout the two-step IVM. In conclusion, these results indicate that CNP pretreatment could dramatically improve the quality of bovine oocytes during in vitro maturation.


Similar content being viewed by others
References
Adhikari D, Liu K (2014) The regulation of maturation promoting factor during prophase I arrest and meiotic entry in mammalian oocytes. Mol Cell Endocrinol 382:480–487
Adona PR, Pires PR, Quetglas MD, Schwarz KR, Leal CL (2008) Nuclear maturation kinetics and in vitro embryo development of cattle oocytes prematured with butyrolactone I combined or not combined with roscovitine. Anim Reprod Sci 104:389–397
Albuz FK, Sasseville M, Lane M, Armstrong DT, Thompson JG, Gilchrist RB (2010) Simulated physiological oocyte maturation (SPOM): a novel in vitro maturation system that substantially improves embryo yield and pregnancy outcomes. Hum Reprod 25:2999–3011
Al-Gubory KH, Fowler PA, Garrel C (2010) The roles of cellular reactive oxygen species, oxidative stress and antioxidants in pregnancy outcomes. Int J Biochem Cell Biol 42:1634–1650
Bierkamp C, Luxey M, Metchat A, Audouard C, Dumollard R, Christians E (2010) Lack of maternal Heat Shock Factor 1 results in multiple cellular and developmental defects, including mitochondrial damage and altered redox homeostasis, and leads to reduced survival of mammalian oocytes and embryos. Dev Biol 339:338–353
Chiaratti MR, Meirelles FV (2010) Mitochondrial DNA copy number, a marker of viability for oocytes. Biol Reprod 83:1–2
Conti M, Andersen CB, Richard F, Mehats C, Chun SY, Horner K, Jin C, Tsafriri A (2002) Role of cyclic nucleotide signaling in oocyte maturation. Mol Cell Endocrinol 187:153–159
Cotterill M, Harris SE, Collado Fernandez E, Lu J, Huntriss JD, Campbell BK, Picton HM (2013) The activity and copy number of mitochondrial DNA in ovine oocytes throughout oogenesis in vivo and during oocyte maturation in vitro. Mol Hum Reprod 19:444–450
Dode MA, Adona PR (2001) Developmental capacity of Bos indicus oocytes after inhibition of meiotic resuption by 6-dimethylaminopurine. Anim Reprod Sci 65:171–180
Eichenlaub-Ritter U, Wieczorek M, Lüke S, Seidel T (2011) Age related changes in mitochondrial function and new approaches to study redox regulation in mammalian oocytes in response to age or maturation conditions. Mitochondrion 11:783–796
Ferreira EM, Vireque AA, Adona PR, Meirelles FV, Ferriani RA, Navarro PA (2009) Cytoplasmic maturation of bovine oocytes: structural and biochemical modifications and acquisition of developmental competence. Theriogenology 71:836–848
Franciosi F, Coticchio G, Lodde V, Tessaro I, Modina SC, Fadini R, Dal Canto M, Renzini MM, Albertini DF, Luciano AM (2014) Natriuretic peptide precursor C delays meiotic resumption and sustains gap junction-mediated communication in bovine cumulus-enclosed oocytes. Biol Reprod 91:61
Franco R, Cidlowski JA (2009) Apoptosis and glutathione: beyond an antioxidant. Cell Death Differ 16:1303–1314
Gharibi S, Hajian M, Ostadhosseini S, Hosseini SM, Forouzanfar M, Nasr-Esfahani MH (2013) Effect of phosphodiesterase type 3 inhibitor on nuclear maturation and in vitro development of ovine oocytes. Theriogenology 80:302–312
Gilchrist RB, Thompson JG (2007) Oocyte maturation: emerging concepts and technologies to improve developmental potential in vitro. Theriogenology 67:6–15
Hardy K, Spanos S, Becker D, Iannelli P, Winston RM, Stark J (2001) From cell death to embryo arrest: mathematical models of human preimplantation embryo development. Proc Natl Acad Sci U S A 98:1655–1660
Hiradate Y, Hoshino Y, Tanemura K, Sato E (2014) C-type natriuretic peptide inhibits porcine oocyte meiotic resumption. Zygote 22:372–377
Hussein TS, Froiland DA, Amato F, Thompson JG, Gilchrist RB (2005) Oocytes prevent cumulus cell apoptosis by maintaining a morphogenic paracrine gradient of bone morphogenetic proteins. J Cell Sci 118:5257–5268
Hwang AB, Jeong DE, Lee SJ (2012) Mitochondria and organismal longevity. Curr Genomics 13:519–532
Iwata H, Goto H, Tanaka H, Sakaguchi Y, Kimura K, Kuwayama T, Monji Y (2011) Effect of maternal age on mitochondrial DNA copy number, ATP content and IVF outcome of bovine oocytes. Reprod Fertil Dev 23:424–432
Kanaya H, Hashimoto S, Teramura T, Morimoto Y, Matsumoto K, Saeki K, Iritani A, Hosoi Y (2007) Mitochondrial dysfunction of in vitro grown rabbit oocytes results in preimplantation embryo arrest after activation. J Reprod Dev 53:631–637
Kang KA, Piao MJ, Kim KC, Cha JW, Zheng J, Yao CW, Chae S, Hyun JW (2014) Fisetin attenuates hydrogen peroxide-induced cell damage by scavenging reactive oxygen species and activating protective functions of cellular glutathione system. In Vitro Cell Dev Biol Anim 50:66–74
Khan M, Yi F, Rasul A, Li T, Wang N, Gao H, Gao R, Ma T (2012) Alantolactone induces apoptosis in glioblastoma cells via GSH depletion, ROS generation, and mitochondrial dysfunction. IUBMB Life 64:783–794
Lopes AS, Lane M, Thompson JG (2010) Oxygen consumption and ROS production are increased at the time of fertilization and cell cleavage in bovine zygotes. Hum Reprod 25:2762–2773
Lord T, Aitken RJ (2013) Oxidative stress and ageing of the post-ovulatory oocyte. Reproduction 146:R217–R227
Marei WF, Wathes DC, Fouladi-Nashta AA (2010) Impact of linoleic acid on bovine oocyte maturation and embryo development. Reproduction 139:979–988
May-Panloup P, Chretien MF, Malthiery Y, Reynier P (2007) Mitochondrial DNA in the oocyte and the developing embryo. Curr Top Dev Biol 77:51–83
Mingoti GZ, Castro VS, Méo SC, Sá Barretto LS, Garcia JM (2011) The effects of macromolecular and serum supplements and oxygen tension during bovine in vitro procedures on kinetics of oocyte maturation and embryo development. In Vitro Cell Dev Biol Anim 47:361–367
Norris RP, Ratzan WJ, Freudzon M, Mehlmann LM, Krall J, Movsesian MA, Wang H, Ke H, Nikolaev VO, Jaffe LA (2009) Cyclic GMP from the surrounding somatic cells regulates cyclic AMP and meiosis in the mouse oocyte. Development 136:1869–1878
Paim LM, Gal LL, Lopes RF, Oliveira AT (2015) Vitrification of Rattus norvegicus immature cumulus-oocyte complexes using hyaluronic acid. In Vitro Cell Dev Biol Anim 51:995–1002
Robinson JW, Zhang M, Shuhaibar LC, Norris RP, Geerts A, Wunder F, Eppig JJ, Potter LR, Jaffe LA (2012) Luteinizing hormone reduces the activity of the NPR2 guanylyl cyclase in mouse ovarian follicles, contributing to the cyclic GMP decrease that promotes resumption of meiosis in oocytes. Dev Biol 366:308–316
Sena LA, Chandel NS (2012) Physiological roles of mitochondrial reactive oxygen species. Mol Cell 48:158–167
Takahashi T, Igarashi H, Amita M, Hara S, Matsuo K, Kurachi H (2013) Molecular mechanism of poor embryo development in postovulatory aged oocytes: mini review. J Obstet Gynaecol Res 39:1431–1439
Vandaele L, Thys M, Bijttebier J, Van Langendonckt A, Donnay I, Maes D, Meyer E, Van Soom A (2010) Short-term exposure to hydrogen peroxide during oocyte maturation improves bovine embryo development. Reproduction 139:505–511
Wu GM, Sun QY, Mao J, Lai L, McCauley TC, Park KW, Prather RS, Didion BA, Day BN (2002) High developmental competence of pig oocytes after meiotic inhibition with a specific M-phase promoting factor kinase inhibitor, butyrolactone I. Biol Reprod 67:170–177
Zamah AM, Hsieh M, Chen J, Vigne JL, Rosen MP, Cedars MI, Conti M (2010) Human oocyte maturation is dependent on LH-stimulated accumulation of the epidermal growth factor-like growth factor, amphiregulin. Hum Reprod 25:2569–2578
Zhang J, Wei Q, Cai J, Zhao X, Ma B (2015) Effect of C-type natriuretic peptide on maturation and developmental competence of goat oocytes matured in vitro. PLoS One 10:e0132318
Zhang M, Su YQ, Sugiura K, Xia G, Eppig JJ (2010) Granulosa cell ligand NPPC and its receptor NPR2 maintain meiotic arrest in mouse oocytes. Science 330:366–369
Zhong Y, Lin J, Liu X, Hou J, Zhang Y, Zhao X (2015) C-type natriuretic peptide maintains domestic cat oocytes in meiotic arrest. Reprod Fertil Dev. doi:10.1071/RD14425
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 31460598) and the Natural Science Foundation of Inner Mongolia Autonomous Region of China (No. 2014MS0327).
Author information
Authors and Affiliations
Corresponding author
Additional information
Editor: Tetsuji Okamoto
Rights and permissions
About this article
Cite this article
Zhang, T., Zhang, C., Fan, X. et al. Effect of C-type natriuretic peptide pretreatment on in vitro bovine oocyte maturation. In Vitro Cell.Dev.Biol.-Animal 53, 199–206 (2017). https://doi.org/10.1007/s11626-016-0101-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11626-016-0101-6