Abstract
Water pollution, evident by negative values of redox potential in waters, occurs at the lagoonal coast located near the densely populated area of Fongafale Islet on Funafuti Atoll, Tuvalu, Central Pacific. Sediment microbial quinone analysis revealed that these coastal sediments exhibit 2.7–10.4 times more microbial biomass, significantly different microbial community structure and low microbial diversity, when compared to an undisturbed natural coastal sediment. Thus, the pollution is chronic. By considering the total land use/coverage on the islet, the situation of septic tank installations, temporal changes in water redox potential and Escherichia coli numbers in the coastal waters and the spatial distribution of acid volatile sulfide in the sediments, we conclude that domestic wastewater is the primary source of pollution. This pollution is proposed to occur via the following mechanism: during ebb tides, domestic wastewater leaking from bottomless septic tanks and pit toilets run off into the lagoonal coast. Tide changes control the pollution load of domestic wastewater.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Anthropogenic pollution in reef-flat seawater is of great concern for coastal conservation. This is because reef island sediments are produced by calcifying organisms, such as coral, coralline algae, molluscs and large benthic foraminifera, that live in the adjacent reefs. In Central Pacific atolls (e.g., Tuvalu, Kiribati, Marshall Islands), shells of large benthic foraminifera are the primary components of sand-sized sediments (Collen and Garton 2004; Yamano et al. 2005). Thus, corals and foraminifera are two major sand producers. Coral reefs on the ocean side act as a natural breakwater and provide bioclastic materials. If a coral reef is healthy without receiving adverse impacts such as rising acidity of seawater, it has an upward growth potential of as much as 400 mm/100 years, which matches the median predicted value of sea-level rise. Thus, a healthy coral reef has the potential to keep up with rising sea level (Kayanne et al. 2005).
Recent studies have suggested that reef islands and adjacent coral reefs located near densely populated areas are being affected by wastewater discharge and waste disposal (Abraham et al. 2004; Richmond et al. 2002; Vieux et al. 2004). The main islands of atoll nations are densely populated (e.g., 8,300 people/km2 on Fongafale, Tuvalu; 2,558 people/km2 on South Tarawa, Kiribati and 11,724 people/km2 on Majuro, Marshall Islands) (Secretariat of the Pacific Community 2005, 2007; Economic Policy, Planning and Statistics Office 2007) owing to limited habitable areas. Concentrations of nutrients were high in reef-flat seawater near densely populated islands, resulting in both direct and indirect negative effects on foraminifera through habitat changes and/or the collapse of algal symbiosis (Osawa et al. 2010). Such reduced water quality on coral reefs caused changes in benthic foraminiferal communities (Hallock et al. 2003; Uthicke and Nobes 2008; Carilli and Walsh 2012). Large benthic foraminifera were rare or absent in the ocean reef flat of Majuro Atoll (Fujita et al. 2009), in lagoons and ocean reef flats of the south Tarawa Atoll (Ebrahim 2000) and in the vicinity of wastewater outfalls on Enewetak Atoll (Hirshfield et al. 1968). The decrease in sediment supply has the potential to contribute to increased coastal erosion (Collen and Garton 2004); however, the mechanisms causing such high nutrient concentrations are as yet unknown.
Reef islands and their populations are considered vulnerable to a range of climatic changes including sea-level rise and similar extreme occurrences (Mimura et al. 2007). The most anticipated physical impacts of sea-level rise on reef islands are shoreline erosion, inundation, flooding, salinity intrusion and reduced resilience of the coastal ecosystem (Khan et al. 2002; Leatherman 1997; Mimura 1999; Yamano et al. 2007). If the atoll nations disappear, there will be no islands left and nothing to inhabit (Connell 2004).
Considering the above studies, a degradation of coral reefs and a decline in large benthic foraminifera, caused by anthropogenic impacts, will accelerate the onset of serious problems that may be caused by future sea-level rise. Therefore, studies are urgently needed to develop and implement countermeasures in order to protect these areas against coastal water pollution. In this paper, we investigate the current water quality of the densely populated lagoonal coasts in Fongafale Islet, Central Pacific and the occurrence of water pollution. We then compare them with less populated natural coast in the islet. The primary pollution sources and pollution mechanism are identified. Through this investigation, we demonstrate the need for effective water quality control measures for coastal conservation.
Materials and methods
Study area
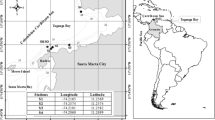
Field surveys were conducted on Fongafale Islet (8°31′S, 179°12′E) in April and August 2010, and January and August 2011. The islet is located on Funafuti Atoll, Tuvalu, a lagoon of ~18 km in diameter (Fig. 1a, b). Fongafale Islet is the capital of Tuvalu and the largest settlement in this country. Approximately 4,492 people live on Funafuti Atoll and 9,561 live in Tuvalu (Secretariat of the Pacific Community 2005). Six sampling points were selected on the lagoon side of Fongafale Islet (Fig. 1c). Site 1 is near the southern tip, where there are no nearby inhabitants. Thus, this site is considered to be very close to an undisturbed natural environment. Sites 2-1, 2-2, 2-3 and 2-4 are along a densely populated area (Yamano et al. 2007). Site 3 is a medium populated area, which is located ~5 km north of site 2-2. All sites are ~15 m from the shore of the lagoonal coast. Surface current flows north-ward along Fongafale Islet at both neap and spring tides and the current speed is less than 0.1 m/s (Damlamian 2008).
Seawater analyses
Water quality measurements
A water quality sonde (Model 6600V2, YSI/Nanotech, Kawasaki, Japan) was installed at ~20 cm from the reef-flat sediment and at 40–60 cm water depth at sites 1, 2-2 and 3, on 5, 3 and 4 April 2010, respectively. Water temperature, electrical conductivity (EC), salinity, dissolved oxygen (DO), pH and redox potential (Eh) were observed routinely at intervals of 10 min for around 1 day on the same days. Further observation was conducted at site 2-2 from 6 to 10 August 2010 at the same intervals for 4 days, in order to investigate the behavior of domestic wastewater runoff.
Escherichia coli
Escherichia coli is a coliform bacterium found most commonly in fecal material, more so than other fecal coliform genera (Metcalf and Eddy 2003). Surface waters were sampled in triplicate (250 mL) at all sites at about 0930 hours (low tide) and at about 1530 hours (high tide) on 27 August 2011. To understand wastewater runoff mechanisms, continuous observation of E. coli was performed every 1–2 h in a similar way at site 2-2 on 7 August 2010 and 29 August 2011. The former observation date was between neap tide and the following spring tide, and the latter was just after spring tide (Fig. 2). The samples were taken immediately to an on-site laboratory and were assayed within 1 h.
Water samples (10 mL) were diluted 10-fold with sterile distilled water and were subjected to most probable number analysis using a commercial test kit (Colilert 18/QuantiTray™, IDEXX Laboratories, Tokyo, Japan) (Fricker et al. 1997). The samples were incubated at 37 °C for 18 h, in accordance with the manufacturer’s instructions.
Sediment analyses
Microbial quinone
Microbial quinone is an essential component in the electron transport chain of microorganisms (Hiraishi et al. 1989). Quinones are divided into two groups: respiratory quinones and photosynthetic quinones. Respiratory quinones, ubiquinone (Q) and menaquinone (MK), exist in bacteria that use respiration to gain energy. In general, ubiquinone is used for aerobic or anoxic respiration and menaquinone for aerobic or anaerobic respiration (Jones 1988). Photosynthetic quinones, plastoquinone (PQ) and vitamin K1 (VK1), are present in photosynthetic microorganisms such as microalgae and cyanobacteria (Collins and Jones 1981; Jones 1988). Each microorganism has only one predominant quinone associated with that species, which is stable even when environmental conditions change. The content of quinone corresponds to the amount of biomass of the microorganisms (Hiraishi et al. 1989). Therefore, quinones have been used as a biomarker to quantitatively analyze a microbial community structure in aqueous environments, such as tidal flats or seabed sediments (Hasanudin et al. 2004, 2005).
It is known that quinone species are assigned to phylogenetic taxa on the basis of the available chemotaxonomic information (Hiraishi et al. 1989). Q-8, Q-9 and Q-10 are assigned to the beta, gamma and alpha subclasses of Proteobacteria, respectively (Yokota et al. 1992). MK-6, MK-7 and MK-8 are assigned to taxonomic groups including the Flavobacterium-Cytophaga group (Nakagawa and Yamasato 1993) and gram-positive bacteria with low G + C contents (Collins and Jones 1981). In addition, MK-7 occurs in sulfate-reducing bacteria such as Desulfotomaculum and Desulfococcus species (Collins and Widdel 1986).
To evaluate microbial community structure, 250 mL surface sediments, up to ~10 cm depth, were sampled at sites 1, 2-2 and 3 on 10 August 2010. Samples were stored at −20 °C. Microbial quinone in the sediments was assayed according to a procedure reported previously (Hasanudin et al. 2004, 2005). Lipids, including quinone, were extracted from the sediment sample with a chloroform–methanol mixture (2:1, v/v) that was re-extracted with hexane. The crude quinone extract in hexane was concentrated using a solid-phase extraction cartridge (Sep-Pak® Plus Silica, Nihon Waters, Tokyo, Japan) and was separated into menaquinone and ubiquinone with 2 and 10 % diethylether–hexane, respectively. Ubiquinone and menaquinone were analyzed using a high-performance liquid chromatography (SCL-10A VP, Shimadzu, Kyoto, Japan) with a photodiode array detector (SPD-M10A, Shimadzu, Kyoto, Japan). Quinone species were identified by their spectrum and the equivalent number of isoprene units (Hiraishi et al. 1989).
Acid volatile sulfides
Sediment samples were collected at up to ~10 cm depth from surface layer of all sites on 20 and 21 January 2011, and the concentration of acid volatile sulfides (AVS) in the sediments was determined in triplicate using an AVS detector tube (210H and 210L, Gastec, Ayase, Japan) following the manufacturer’s instructions.
Statistical analyses
Microbial dissimilarity
To investigate the quantitative differences in the microbial community structure based on respiratory quinone in the sediments, a dissimilarity index value (D-value) was calculated using Eq. (1) (Hiraishi et al. 1991):
where n is the number of quinone species and f i,k and f j,k are the molar fractions of quinone species k for any two samples i and j, respectively. The D-value ranged from 0 to 1. The values greater than 0.2 were interpreted as having a significant difference in the microbial community (Hiraishi et al. 1991). To visually understand microbial dissimilarity among all the sediment samples, multidimensional scaling (MDS) and cluster analysis with an unweighted pair group method using arithmetic averages were carried out on the basis of the quinone fraction using a statistical package (PASW® Statistics 18, SPSS Japan, Tokyo, Japan). The Kruskal’s stress and R 2 measures are used to test the reliability and validity of the MDS results; Kruskal’s stress is the measure most commonly used for determining the MDS model’s badness of fit. Kruskal and Wish (1978) give the following numbers as guideline: 0.00 a perfect fit, 0.025 an excellent fit, 0.05 a good fit, 0.10 a fair fit and 0.20 a poor fit. An R 2 of 0.6 is considered the minimum acceptable level for the validity of the MDS analysis.
Microbial diversity
To evaluate microbial diversity in terms of the richness and evenness of the quinone species, Shannon–Wiener diversity H′ was estimated according to Eq. (2) (Shannon and Weaver 1963):
where n is the number of quinone species and f k is the molar fraction of quinone species k for a sample. Typically, the value ranged from 1.5 to 3.5, indicating a low to high richness and evenness of species.
Results and discussion
Water pollution status
Water quality
Average EC and salinity at site 1 were 52.8 mS/cm and 34.7 ‰, respectively, which are comparable to values of natural seawater (Fig. 3). A temporary drop in EC and salinity was found at about 0800 hours on 6 April because of rainfall. The values at sites 2-2 and 3 were slightly lower than those values at site 1 and then lower than those of natural seawater. This is probably a result of the mixing of coastal waters with waters possessing low EC and salinity, excluding rainwater, since no significant rainfall occurred during the observation period.
DO and pH ranged from 4.5 to 7.2 and from 8.1 to 8.3 at site 1, respectively. Site 2-2 and site 3 in particular displayed more variation. DO and pH decreased during the night and increased during the day. These variations are likely in response to respiration and photosynthesis by photosynthetic microorganisms.
Surprisingly, negative Eh values were found at sites 2-2 and 3, whilst site 1 showed positive values during the entire observational period. Site 2-2 displayed quite a different trend to that of site 3. The minimum Eh value of −61 mV appeared at midnight at site 2-2, although the trend of variation in Eh was quite similar to those in DO and pH at site 3. From the results, there is a possibility that wastewater flows into the coastal area at site 2-2.
Sediment microbial community structure
Plastoquinone with nine isoprene units (PQ-9) and VK1 were detected at 0.25 and 0.14 μmol/kg in total at sites 2-2 and 3, respectively, but 0.04 μmol/kg at site 1 (Table 1). The contents at sites 2-1 and 2-3 were also similar to or greater than that at site 3, indicating the presence of sufficient nutrients at these sites to maintain a higher abundance of photosynthetic microorganisms.
At site 1, the respiratory quinone content in the sediment sample was 0.04 μmol/kg, composed of ubiquinone and menaquinone (Fig. 4). On the other hand, the quinone content at sites 2-1, 2-2, 2-3 and 2-4 ranged from 0.14 to 0.54 μmol/kg and that at site 3 was 0.27 μmol/kg. The sediments near the populated areas had a microbial biomass 2.7–10.4 times that of the unpolluted area sediment. The higher microbial biomass suggests that the organic matter and nutrients used for their growth in sediment are supplied to the four sites, particularly site 2-2, by the coastal communities.
At site 1, the most predominant quinone species was ubiquinone with eight isoprene units (Q-8), followed by menaquinone with six isoprene units (MK-6) and MK-8. The order of occurrence of the units at sites 2-1, 2-2, 2-3 and 2-4 was Q-8 > Q-9 or Q-10 or MK-7 > Q-9 or MK-7 or MK-8 and that at site 3 was Q-8 > Q-10 > MK-7. MK-7 has been detected as the second or third major quinone species at these sites near the populated area, indicating the presence of sulfate-reducing bacteria. This is also indicated by the occurrence of negative Eh values in the coastal waters at site 2-2 and the probable presence of organic matter and nutrients in the coastal areas at sites 2-1, 2-2, 2-3 and 2-4, and site 3.
Differences seen in the major quinone species indicate that bacteria of different taxonomic groups inhabit the sediments. To quantitatively identify the differences in the microbial community structure based on respiratory quinone, D-values were calculated and subjected to MDS and cluster analyses. The stress value and R 2 value were estimated to be 0.14 and 0.95, respectively, indicating an acceptable level for the fit and validity of the MDS analysis. These analyses categorized the six sites into four groups: site1, sites 2-1, 2-2, 2-3 and 2-4, and site 3 (Fig. 5a, b). This indicates that the microbial community structures were similar at sites 2-1, 2-2, 2-3 and 2-4, and significantly different from that of site1. The microbial community structure at site 3 is also distinct from that of site 1. The Shannon–Wiener diversity values at sites 2-1, 2-2, 2-3 and 2-4, and site 3 were relatively low compared to those at site 1 (Fig. 6). This is because specific bacteria, such as Q-8-containing proteobacterial species, were significantly predominant at sites 2-1, 2-2, 2-3 and 2-4, and site 3 although the abundance of the number of quinone species was similar at the other sites. These results indicate that coastal sediments near populated areas tend to have pockets of sediments with high contents of organic matter and nutrients. Generally, bioindicators are used for the evaluation of long-term environmental impacts. Thus, this study indicates that water pollution is a chronic problem on the lagoon side of the island near the populated area, also taking into account the high density of population.
Water pollution mechanism
Water pollution sources
Considering the land use/coverage on Fongafale Islet (Yamano et al. 2007), it is unlikely that non-point source pollution and/or industrial wastewater were the primary sources of pollution. Fongafale Islet has 639 households (Secretariat of the Pacific Community 2005). Although there is no centralized treatment system such as a wastewater treatment plant, 424 households have buried septic tanks that receive domestic wastewaters including human waste. Specifications require the septic tank to have two compartments: one for settling and one for anaerobic treatment. In addition, 163 households have pit toilets with a pour flush (Secretariat of the Pacific Community 2005; Lal et al. 2006). Thus, 92 % of households have access to improved sanitary facilities. However, studies have shown that septic tank systems (Borchardt et al. 2003; DeWalle and Schaff 1980; Scandura and Sobsey 1997; Viraghaven and Warnock 1976) and pit toilets (Dzwairo et al. 2006; Montgomery and Elimelech 2007; Pedley and Howard 1997) are a source of groundwater contamination. Thus, the disposal of human waste using these facilities is a key issue for groundwater quality and public health protection. The Public Works Department of the Tuvalu government was surveyed about the design and integrity of the septic tanks on the islet. Surprisingly, it was determined that the bottoms of the septic tanks were not sealed—so called ‘bottomless’. Construction specifications proposed by Australia require these tanks to be sealed; however, these tanks were constructed with a disregard for these specifications. Thus, considering also the fact that the Holocene sand aquifer with high permeability extends from the surface to the depth of ~ 20 m on Fongafale Islet (Ohde et al. 2002), the potential sources of pollution of the lagoon side coast are bottomless septic tanks and pit toilets.
Wastewater runoff mechanism
Nakada et al. (2012) reported ground water dynamics in the lagoonal coast using electrical resistivity. Saline water extended landward from the coastal area during flood tides, and brackish water receded coastward from the inland area during ebb tides. This indicates that if there are leaks from bottomless septic tanks and pit toilets, they subsequently flow into the coastal lagoon. The Eh value should then respond and fecal indicator bacteria, E. coli, would be detected, because the wastewater includes human waste.
As shown in Fig. 7, periodic variations were observed in Eh. The timing of these variations was similar to that of the sea level data obtained from the National Tidal Centre (2010). A periodic variation is observed during the whole tidal cycle. The Eh became more negative during ebb tides and then gradually became more positive with time. Salinity and EC also showed similar trends (data not shown). The observational period was during the transition from neap tide to spring tide; thus, the Eh increase is presumably due to the ongoing of water exchange between the lagoon and the ocean.
At low tide, the number of E. coli was 1.1 × 10 MPN/100 mL at site 1; however, E. coli numbers ranged from 3.2 × 103 to 2.7 × 104 MPN/100 mL at sites 2-1, 2-2, 2-3 and 2-4, and reached 6.2 × 10 MPN/100 mL at site 3 (Fig. 8). At high tide, E. coli was not detected at site 1 and site 3. Sites 2-1, 2-2, 2-3 and 2-4 ranged from 5.5 × 102 to 1.2 × 103 MPN/100 mL. High numbers of E. coli were found at sites 2-1, 2-2, 2-3 and 2-4 compared to site 1 and site 3, and higher values were found at low tide than at high tide. Japanese water quality criteria stipulate that the number of colon bacilli should not exceed 1.0 × 103 MPN/100 mL for bathing beaches. Since E. coli forms part of colon bacillus species, such high numbers of E. coli in the coastal waters pose concerns as a human health risk. The Eh and E. coli results indicate that ebb tides enable domestic wastewater to flow through groundwater into the coastal waters. This is also supported by sediment analysis.
As shown in Fig. 9, relatively high numbers of E. coli were detected during the ebb tide on 7 August 2010 and 29 August 2011. However, the maximum number of E. coli during the ebb tide on 7 August 2010 was 2.5 × 104 MPN/100 mL, while it was 1.1 × 103 MPN/100 mL on 29 August 2011. The transition period from neap tide to spring tide would gradually increase the amount of sea water flowing into the septic tank from the bottom and the amount of domestic wastewater leaking and subsequently flowing into the coastal waters, due to the gradual increase in water-level difference between high tide and low tide. On the other hand, just after a spring tide, domestic wastewater inside the septic tanks would mostly have leaked out, because of the maximum water-level difference. Thus, high numbers of E. coli as observed on 7 August 2010 would not be found. These runoff mechanisms give the explanation of the differences in the numbers of E. coli on 7 August 2010 and 29 August 2011.
Surficial sediments at sites 2-1, 2-2, 2-3 and 2-4 were grey sand with a hydrogen sulfide odour. AVS concentrations ranged from 0.024 to 0.133 mg/g. This corresponds to the sediment quinone analysis that detected MK-7, which occurs in sulfate-reducing bacteria. Digging in the sandy beach between the households and the coast revealed similar grey sand. However, no grey sand was found at the other sites and AVS concentrations were less than the detection limit (0.002 mg/g). Therefore, sulfate reduction occurs in sediments from sites 2-1, 2-2, 2-3 and 2-4. This further lends support to the hypothesis that domestic wastewater runoff migrates to the coast through the groundwater.
There is a strong possibility that the coastal water pollution in the lagoon due to poorly constructed sanitary facilities is connected to the decrease in sand supply as observed in other atolls (Ebrahim 2000; Fujita et al. 2009; Hirshfield et al. 1968), because the coastal environments are chronically damaged. In other words, the results from this study demonstrate an urgent need for the development and implementation of effective water quality control strategies. To consider such strategies, we should pay attention to both hard and soft infrastructures. The former in order to improve the purification capability of existing sanitary facilities for wastewater treatment. Improved sanitary facilities should be suitable for the geophysical and social surroundings specific to atolls. The latter in order to establish a policy for the water quality improvement and to develop local capacity building. We have reported the current status and mechanism of the lagoonal water pollution to the Tuvalu government. Government officials have deep concerns about the serious situation and their Tuvaluan counterparts are working on a proposal for a project based on our results to improve remediation of water pollution. Our scientific results are being utilized by working together. On the other hand, we have trained them in skills for water quality assays so they can get by on their own. We very much hope that our work finally connects with their policy decisions, and that this will become a good example of working practice because many atolls are facing a similar situation due to either installation of similar sanitary facilities or no treatment of wastewater.
Conclusions
Coastal water pollution of atolls due to human impacts has long been recognized (e.g., Johannes et al. 1979; Kimmerer and Walsh 1981). This paper has demonstrated water pollution mechanisms in lagoonal coasts for the first time by surveying near the densely populated area of Fongafale Islet on Funafuti Atoll, Tuvalu. Water pollution is a chronic problem, and domestic wastewater is cited as the primary pollution source. This occurs even though 92 % of households have access to improved sanitary facilities such as septic tanks and pit toilets. However, this study determined that these so called ‘improved sanitary facilities’ were not built as per the design specifications or they are not suitable for the geophysical characteristics. Although the septic tanks should be sealed at the bottom, many of the tanks within the study area were not sealed. Thus, during ebb tides, domestic wastewater leaking from bottomless septic tanks and pit toilets runs off into coastal waters. Tide changes control the pollution load of domestic wastewater.
References
Abraham T, Beger M, Burdick D, Cochrane E, Craig P, Didonato G, Fenner D, Green A, Golbuu Y, Gutierrez J, Hasurmai M, Hawkins C, Houk P, Idip D, Jacobson D, Joseph E, Keju T, Kuartei J, Palik S, Penland L, Pinca S, Rikim K, Starmer J, Trianni M, Victor S, Whaylen L (2004) Status of the coral reefs in Micronesia and American Samoa. In: Wilkinson C (ed) Status of Coral Reefs of the World: 2004. Australian Institute of Marine Science, Townsville, pp 381–410
Borchardt MA, Bertz PD, Spencer SK, Battigelli DA (2003) Incidence of enteric viruses in groundwater from household wells in Wisconsin. Appl Environ Microbiol 69:1172–1180
Carilli J, Walsh S (2012) Benthic foraminiferal assemblages from Kiritimati (Christmas) Island indicate human-mediated nutrification has occurred over the scale of decades. Mar Ecol Prog Ser 456:87–99
Collen JD, Garton DW (2004) Larger foraminifera and sedimentation around Fongafale Island, Funafuti Atoll, Tuvalu. Coral Reefs 23:445–454
Collins MD, Jones D (1981) Distribution of isoprenoid quinone structural types in bacteria and their taxanomic implications. Microbiol Rev 45(2):316–354
Collins MD, Widdel F (1986) Respiratory quinones of sulphate-reducing and sulphur-reducing bacteria: a systematic investigation System. Appl Microbiol 8(1–2):8–18
Connell J (2004) Environmental change, economic development, and emigration in Tuvalu. In: Lockwood VS (ed) Globalization and culture change in the Pacific Islands. Pearson, Prentice Hall, Upper Saddle River, NJ, pp 260–272
Damlamian H (2008) Hydrodynamic model of Funafuti: water circulation and applications. EU EDF–SOPAC Project Report 133, Fiji
DeWalle FB, Schaff RM (1980) Ground-water pollution by septic tank drainfields. J Environ Eng Div 106:631–646
Dzwairo B, Hoko Z, Love D, Guzha E (2006) Assessment of the impacts of pit latrines on groundwater quality in rural areas: a case study from Marondera district Zimbabwe. Phys Chem Earth 31(15–16):779–788
Ebrahim MT (2000) Impact of anthropogenic environmental change on larger foraminifera: Tarawa Atoll, Kiribati, South Pacific. In: Martin RE (ed) Environmental micropaleontology. Kluwer/Plenum, New York, pp 105–119
Economic Policy, Planning and Statistics Office (EPPSO) (2007) Republic of the Marshall Islands Demographic and Health Survey 2007. SPC and Macro International, Noumea
Fricker EJ, Illingworth KS, Fricker CR (1997) Use of two formulations of Colilert and QuantiTray™ for assessment of the bacteriological quality of water. Water Res 31(10):2495–2499
Fujita K, Osawa Y, Kayanne H, Ide Y, Yamano H (2009) Distribution and sediment production of large benthic foraminifers on reef flats of the Majuro Atoll, Marshall Islands. Coral Reefs 28:29–45
Hallock P, Lidz BH, Cockey-Burkhard EM, Donnelly KB (2003) Foraminifera as bioindicators in coral reef assessment and monitoring: the FORAM index. Environ Monit Assess 81:221–238
Hasanudin A, Fujita M, Kunihiro T, Fujie K, Suzuki T (2004) The effect of clams (Tapes philippinarum) on changes in microbial community structure in tidal flat sediment mesocosms, based on quinone profiles. Ecol Eng 22:185–196
Hasanudin A, Fujita M, Koibuchi Y, Fujie K (2005) Dynamic changes in environment condition and microbial community structure in trench and flat seabed sediments of Tokyo Bay, Japan. Water Sci Technol 52(9):107–114
Hiraishi A, Masamune K, Kitamura H (1989) Characterization of the bacterial population structure in an anaerobic-aerobic activated sludge system on the basis of respiratory quinone profiles. Appl Environ Microbiol 55(4):897–901
Hiraishi A, Morishima Y, Takeuchi J (1991) Numerical analysis of lipoquinone pattern in monitoring bacterial in wastewater treatment systems. J Gen Appl Microbiol 37:57–70
Hirshfield HI, Charmatz R, Helson L (1968) Foraminifera in samples taken mainly from Eniwetok Atoll in 1956. J Protozool 15:497–502
Johannes R, Kimmerer W, Kinzie R, Shirona E, Walsh TW (1979) The impact of human activities on Tarawa lagoon. SPC, Noumea
Jones CW (1988) Membrane-associated energy conservation in bacteria; a general introduction. In: Anthony C (ed) Bacterial energy transduction. Academic, London, pp 42–46
Kayanne H, Chikamori M, Yamano H, Yamaguchi T, Yokoki H, Shimazaki H (2005) Interdisciplinary approach for sustainable land management of atoll islands. Global Environ Res 9(1):1–7
Khan TMA, Quadir DA, Murty TS, Kabir A, Aktar F, Sarker MA (2002) Relative sea level changes in Maldives and vulnerability of land due to abnormal coastal inundation. Mar Geodesy 25:133–143
Kimmerer WJ, Walsh TW (1981) Tarawa Atoll lagoon: circulation, nutrient fluxes and the impact of human waste. Micronesica 17:161–179
Kruskal JB, Wish M (1978) Multidimensional scaling. Sage Publications, Beverley Hills
Lal P, Saloa K, Uili F (2006) Economics of liquid waste management in Funafuti, Tuvalu, IWP-Pacific Technical Report no. 36, SPREP, Samoa
Leatherman SP (1997) Island states at risk: global climate change, development and population. Coastal Education Research Foundation, Florida
Metcalf and Eddy (2003) Watewater engineering: treatment and reuse, 4th edn. Mc Graw-Hill, Boston
Mimura N (1999) Vulnerability of island countries in the South Pacific to sea level rise and climate change. Clim Res 12:137–143
Mimura N, Nurse L, McLean RF, Agard J, Briguglio L, Lefale P, Payet R, Sem G (2007) Small islands. In: Parry ML, Canziani OF, Palutikof JP, van der Linden PJ, Hanson CE (eds) Climate change 2007: impacts, adaptation and vulnerability. Contribution of Working Group II to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press, Cambridge, pp 687–716
Montgomery MA, Elimelech M (2007) Water and sanitation in developing countries: including health in the equation. Environ Sci Technol 41:17–24
Nakada S, Umezawa Y, Taniguchi M, Yamano H (2012) Groundwater dynamics of Fongafale islet, Funafuti atoll Tuvalu. Ground Water 50:639–644. doi:10.1111/j.1745-6584.2011.00874.x
Nakagawa Y, Yamasato K (1993) Phylogenetic diversity of the genus Cytophaga revealed by 16S rRNA sequencing and menaquinone analysis. J Gen Microbiol 139:1155–1161
National Tidal Centre (2010) Hourly sea level and meteorological data: 2010, south pacific sea level and climate monitoring project. Bureau of Meteorology, Australian Government. http://www.bom.gov.au/ntc/IDO70006/IDO70006_2010.csv
Ohde S, Greaves M, Masuzawa T, Buckley HA, Van Woesik R, Wilson PA, Pirazzoli PA, Elderfield H (2002) The chronology of Funafuti Atoll: revisiting an old friend. Proc R Soc Lond Ser A 458:2289–2306
Osawa Y, Fujita K, Umezawa Y, Kayanne H, Ide Y, Nagaoka T, Miyajima T, Yamano H (2010) Human impacts on large benthic foraminifers near a densely populated area of Majuro Atoll, Marshall Islands. Mar Pollut Bull 60:1279–1287
Pedley S, Howard G (1997) The public health implications of microbiological contamination of groundwater. Q J Eng Geol 30:179–188
Richmond R, Kelty R, Craig P, Emaurois C, Green A, Birkeland C, Davis G, Edward A, Golbuu Y, Gutierrez J, Houk P, Idechong N, Maragos J, Paulay G, Starmer J, Tafileichig A, Trianni M., Velde NV (2002) Status of the coral reefs in Micronesia and American Samoa: US affiliated and freely associated islands in the Pacific. In Wilkinson CR (ed) Status of Coral Reefs of the World: 2002. GCRMN Report. Australian Institute of Marine Science, Townsville, pp 217–236
Scandura JE, Sobsey MD (1997) Viral and bacterial contamination of groundwater from on-site sewage treatment systems. Water Sci Technol 35:141–146
Secretariat of the Pacific Community (2005) Tuvalu 2002 Population and housing census: volume 1 analytical report. Secretariat of the Pacific Community, Noumea
Secretariat of the Pacific Community (2007) Kiribati 2005 census: volume 2 analytical report. Secretariat of the Pacific Community, Noumea
Shannon CE, Weaver W (1963) The mathematical theory of communications. University of Illinois Press, Urbana
Uthicke S, Nobes K (2008) Benthic Foraminifera as ecological indicators for water quality on the Great Barrier Reef. Estuar Coast Shelf Sci 78:763–773
Vieux C, Aubanel A, Axford J, Chancerelle Y, Fisk D, Holland P, Juncker M, Kirata T, Kronen M, Osenberg C, Pasisi B, Power M, Salvat B, Shima J, Vavia V (2004) A century of change in coral reef status in southeast and central Pacific: Polynesia Mana Node, Cook Islands, French Polynesia, Kiribati, Niue, Tokelau, Tonga, Wallis and Futuna. In: Wilkinson C (ed) Status of coral reefs of the world: 2004. Australian Institute of Marine Science, Townsville, pp 363–380
Viraghaven T, Warnock R (1976) Groundwater quality adjacent to a septic tank system. J Am Water Works Assoc 68:611–614
Yamano H, Kayanne H, Chikamori M (2005) An overview of the nature and dynamics of reef islands. Global Environ Res 9:9–20
Yamano H, Kayanne H, Yamaguchi T, Kuwahara Y, Yokoki H, Shimazaki H, Chikamori M (2007) Atoll island vulnerability to flooding and inundation revealed by historical reconstructions: Fongafale Islet, Funafuti Atoll, Tuvalu. Global Planet Change 57:407–416
Yokota A, Akagawa-Matsushita M, Hiraishi A, Katayama Y, Urakami T, Yamasato K (1992) Distribution of quinone systems in microorganisms: gram-negative eubacteria. Bull Jpn Fed Culture Collect 8:136–171
Acknowledgments
The authors would like to thank Mr. Yoichi Ide (Oceanic Planning Corporation, Japan) for the AVS measurement and Dr. Murray Ford (The University of Auckland, New Zealand) for English language review and informative comments on the early version of this manuscript. This research was supported by JST/JICA SATREPS (0808918), Ibaraki University ICAS Research Project, JSPS KAKENHI (24560658), and JGC-S Scholarship Foundation Grant for Young Researchers.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handled by John E. Hay, Ibaraki University, Japan.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Fujita, M., Suzuki, J., Sato, D. et al. Anthropogenic impacts on water quality of the lagoonal coast of Fongafale Islet, Funafuti Atoll, Tuvalu. Sustain Sci 8, 381–390 (2013). https://doi.org/10.1007/s11625-013-0204-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11625-013-0204-x