Abstract
Background
The high prevalence of chronic diseases, including congestive heart failure (CHF), chronic obstructive pulmonary disease (COPD), and diabetes mellitus (DM), accounts for a large burden of cost and poor health outcomes in US hospitals, and home telehealth (HT) monitoring has been proposed to improve outcomes.
Objective
To measure the association between HT initiation and 12-month inpatient hospitalizations, emergency department (ED) visits, and mortality in veterans with CHF, COPD, or DM.
Design
Comparative effectiveness matched cohort study.
Patients
Veterans aged 65 years and older treated for CHF, COPD, or DM.
Main Measures
We matched veterans initiating HT with veterans with similar demographics who did not use HT (1:3). Our outcome measures included a 12-month risk of inpatient hospitalization, ED visits, and all-cause mortality.
Key Results
A total of 139,790 veterans with CHF, 65,966 with COPD, and 192,633 with DM were included in this study. In the year after HT initiation, the risk of hospitalization was not different in those with CHF (adjusted odds ratio [aOR] 1.01, 95% confidence interval [95%CI] 0.98–1.05) or DM (aOR 1.00, 95%CI 0.97–1.03), but it was higher in those with COPD (aOR 1.15, 95%CI 1.09–1.21). The risk of ED visits was higher among HT users with CHF (aOR 1.09, 95%CI 1.05–1.13), COPD (1.24, 95%CI 1.18–1.31), and DM (aOR 1.03, 95%CI 1.00–1.06). All-cause 12-month mortality was lower in those initiating HT monitoring with CHF (aOR 0.70, 95%CI 0.67–0.73) and DM (aOR 0.79, 95%CI 0.75–0.83), but higher in COPD (aOR 1.08, 95%CI 1.00–1.16).
Conclusions
The initiation of HT was associated with increased ED visits, no change in hospitalizations, and lower all-cause mortality in patients with CHF or DM, while those with COPD had both higher healthcare utilization and all-cause mortality.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
More than 60% of adults have been diagnosed with at least one chronic medical condition, and those with chronic conditions comprise approximately 90% of total US healthcare spending.1 Congestive heart failure (CHF), chronic obstructive pulmonary disease (COPD), and diabetes mellitus (DM) rank in the top 10 principal diagnoses for inpatient stays in the USA, accounting for over 2.3 million hospitalizations each year.2 Thirty-day hospital readmissions are estimated at 18% for CHF, 22% for COPD, and 14–22% for DM.3,4,5 Care coordination, patient education, and rapid post-admission follow-up all reduce hospital readmissions, but these services are not uniformly available.6,7 In one study of newly diagnosed CHF patients, those who lived in rural areas had 11% fewer emergency department (ED) visits, 22% fewer hospitalizations, and 18% higher all-cause mortality than those who lived in urban areas, suggesting that unmet healthcare needs contribute to higher mortality..8
Telehealth has been proposed as one low-cost strategy to improve access to care in both acute and chronic conditions.9,10,11 Telehealth enables patients to access healthcare from home with minimal effort and allows for chronic disease management to occur more frequently. In chronic CHF management, for instance, home telehealth (HT) can enable daily weight and vitals monitoring, timely medication changes, provider consultation, and early recognition of decompensation with appropriate intervention.12 Similar programs have been developed for symptom and spirometry monitoring in COPD and blood sugar monitoring in CHF.
The Veterans Health Administration (VHA) has had a HT program for the past 20 years.13 The program involves the use of a set of Disease Management Protocols by a nurse care coordinator in conjunction with daily HT monitoring and regular symptom questionnaires.14 In previous single-center implementation studies, HT monitoring was associated with reduced hospital admissions and decreased costs.15,16,17,18 Telehealth programs function differently at scale, however, so the effect of a HT program on overall population health remains unclear. The objective of this study was to measure the effect of HT use on clinical outcomes and healthcare utilization in a cohort of veterans with chronic disease in a mature telehealth system.
METHODS
Study Design, Population, and Data Sources
This analysis was a multicenter retrospective matched cohort study of veterans ≥ 65 years with CHF, COPD, or DM between 2013 and 2018. The Veterans Health Administration operated a comprehensive national health system including inpatient and outpatient care for veterans throughout the USA. Participants were included if they had vested VHA health coverage (i.e., fully qualified for VA services), at least one primary care visit within the VHA between 2013 and 2018 and an inpatient or outpatient and diagnosis code consistent with CHF, COPD, or DM (Supplemental Table 1).19 We excluded patients with visits in multiple Veterans Integrated Service Networks (VISNs) during the study period, a first HT monitoring code listed after a death date (indicating an administrative error), or missing gender, home location, or Care Assessment Need (CAN) risk adjustment score. The CAN score is a validated risk score that incorporates predictors from sociodemographic variables, medical conditions, vital signs, prior healthcare use, medications, and laboratory tests to estimate the probability of hospital admission or death within 1 year.20,21.
We used VHA administrative records stored in the inpatient and outpatient tables of the VA Informatics and Computing Infrastructure Corporate Data Warehouse for the period 2012–2019 (to allow for a 12-month lookback period and 12-month outcome period), and we defined VA health coverage from the VA Enrollment File. We used records linked with Medicare Part A and B fee-for-service claims in the VA Medicare Analysis Center to identify healthcare utilization outside the VA health system, and we verified vital statistics with the National Death Index.22 The local institutional review board determined that this activity constituted quality improvement, and this manuscript is reported in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement.23 Detailed statistical methods and analytic approaches are described in Supplemental Appendix A.
Exposure
The primary exposure for this analysis was new HT use, defined using VHA-specific codes indicating telehealth (Supplemental Table 2). HT is a system of chronic disease management that incorporates the use of telehealth-enabled monitoring devices (e.g., scales, blood pressure monitoring, digital symptom inventories) that transmit personal health data to a care coordinator.24,25,26 Data are reviewed by a care coordinator or patient’s healthcare provider to recommend changes in home management. These processes are part of a mature chronic disease management system available to veterans with complex chronic health problems and for whom frequent travel for treatment or appointments might be inconvenient.27,28 Supplemental Appendix B (CHF), Supplemental Appendix C (COPD), and Supplemental Appendix D (DM) detail the Disease Management Protocols that are used, and these protocols are available for use in all VA facilities and VISNs nationally. We only included our HT cohort within the first year that patients participated in the program.
Outcomes
The primary outcome of this study was an all-cause inpatient admission in the 12 months after telehealth initiation for exposed veterans and the proxy date for matched unexposed veterans. Secondary outcomes included (1) ED visits and (2) mortality within 12 months. Mortality was defined as all-cause mortality, derived from the VA/Department of Defense Mortality Data Repository.29 Inpatient admissions and ED visits were separately identified in VHA and non-VHA (using Medicare claims) facilities. In the primary analyses, inpatient admission and ED visit outcomes included combined utilization measures across both VHA and non-VHA facilities.
Data Availability
The datasets analyzed during the current study are not publicly available due to data use provisions related to confidentiality within the Veterans Health Administration, but are available from the corresponding author to appropriately credentialed Veterans Administration researchers on reasonable request.
Statistical Analysis
Main Analysis
HT-exposed veterans were matched in a 1:3 ratio with non-HT controls using an exact matching algorithm on age, sex, race/ethnicity, and rurality. To measure the association between HT utilization and each outcome, we used conditional logistic regression for the matched units to assess the unadjusted odds ratio and 95% confidence intervals (CI). We then constructed multivariable models to adjust for demographic and clinical characteristics beyond the matched design variables that might confound the relationship (further explained in Supplemental Appendix A: including service-connected disability percentage, VISN, duration since diagnosis of chronic disease, zip code mean household income, marital status, weighted Elixhauser score for previous 12 months, number of inpatient admissions in previous 12 months [0, 1, > 1], emergency department visit in previous 12 months, CAN score). Hospitalization CAN scores were included for inpatient admission and ED utilization models, while mortality CAN scores were included in the mortality model. Continuous variables including income, CAN scores, weighted Elixhauser comorbidity index, and duration of illness were dichotomized at the median for final model inclusion.
Sensitivity Analyses
We conducted several sensitivity analyses to account for effect modification due to sample and temporal measures. For inpatient admissions and ED utilization, we conducted sensitivity analyses on VA-only healthcare utilization and Medicare-only healthcare utilization to assess the impact of VHA services on the location of care. We measured the association between HT and each outcome separately for rural and urban patients. To measure temporal changes in HT delivery, we measured the association between HT use and each outcome in the 2013–2014 and 2017–2018 cohorts. Finally, to limit survival bias, we conducted a sub-analysis of healthcare utilization outcomes for those who were alive at the end of the 12-month follow-up for each condition.
RESULTS
Participant Characteristics
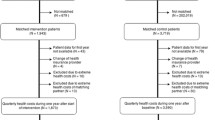
A total of 1,054,630 veterans with CHF, 1,887,915 veterans with COPD, and 2,506,110 veterans with DM were treated between 2013 and 2018, of which 644,019 (61%), 1,422,812 (75%), and 1,662,860 (66%) were eligible for inclusion, respectively. In the final matched sample, there were 34,854 HT users among CHF patients (Supplemental Figure 1), 16,841 HT users among COPD patients (Supplemental Figure 2), and 48,002 HT users among DM patients (Supplemental Figure 3). For each condition, the number of new HT patients declined over the study period (Supplemental Figure 4).
Over 98% of the sample for all three conditions were male, and the majority for each condition were 65–74 years of age (Table 1). In the CHF cohort, those who used HT were more likely to have had ≥ 2 hospital admissions (36% vs. 13%; Cohen’s d effect size 0.57) and an ED visit (51% vs. 29%; effect size 0.44) in the prior year compared to the non-HT cohort, but other demographic and clinical characteristics were similar (Table 2). Similarly for COPD, those who used HT were also more likely to have had ≥ 2 hospital admissions (30% vs. 8%; effect size 0.60) and an ED visit (46% vs. 25%; effect size 0.45) in the prior year compared to the non-HT cohort.
Inpatient Admissions
CHF
In the year after HT initiation, a greater proportion of veterans who used HT had an inpatient admission compared to those without HT [(50% vs. 31%, respectively), unadjusted odds ratio (uOR): 1.45; 95%CI: 1.40–1.50)] (Fig. 1), but this association disappeared in the adjusted model (adjusted odds ratio [aOR] 1.01; 95%CI 0.98–1.05). Those admissions were more likely to occur in a VHA hospital (aOR 1.23; 95%CI 1.18–1.28) (Supplemental Figure 5).
Inpatient admissions of matched cohort by chronic health condition and telehealth status. Each bar shows the proportion of veterans with each chronic condition that had an inpatient admission (left panel). Gray bars show the proportion of veterans that did not use any home telehealth (HT) monitoring that had an inpatient admission during the year, and black bars show the proportion of veterans who had HT monitoring that had an inpatient admission visit during the year. Veterans using both clinical video telehealth and home telemonitoring in any given year were excluded from the sample. In the second panel (right), the unadjusted (gray) and adjusted (black) odds ratios are presented to show the relationship between HT monitoring and inpatient admissions.
COPD
HT was associated with increased admissions in COPD patients (47% vs. 23%, respectively; uOR: 2.98; 95%CI: 2.86–3.09) (Fig. 1). In the adjusted model, this translated to a 15% increase in the odds of inpatient admissions in HT patients (aOR: 1.15; 95%CI: 1.09–1.21). These findings were similar across most sensitivity analyses (Supplemental Figure 6).
DM
Veterans using HT had more admissions compared to those without HT (30% vs. 18%, respectively; uOR: 1.87; 95%CI: 1.83, 1.92) (Fig. 1), but this effect disappeared in multivariable modeling (aOR: 1.00; 95%CI: 0.97–1.03). Similar to CHF, those admissions were more likely to occur in VHA hospitals (aOR: 1.21; 95%CI: 1.16–1.26) (Supplemental Figure 7).
ED Visits
CHF
Veterans who used HT had a greater probability of ED visits compared to those who did not (40% vs. 31%, respectively; uOR: 1.55; 95%CI: 1.51–1.59) (Fig. 2), and this effect persisted in the adjusted model (aOR: 1.09; 95%CI: 1.05–1.13). These increased visits were most pronounced in VHA EDs (aOR: 2.79; 95%CI: 2.62–2.98) (Supplemental Figure 8).
Emergency department visits of matched cohort by chronic health condition and telehealth status. Each bar shows the proportion of veterans with each chronic condition that had an emergency department (ED) visit (left panel). Gray bars show the proportion of veterans that did not use any home telehealth (HT) monitoring that had an ED visit during the year, and black bars show the proportion of veterans who had HT monitoring that had an ED visit during the year. Veterans using both clinical video telehealth and home telemonitoring in any given year were excluded from the sample. In the second panel (right), the unadjusted (gray) and adjusted (black) odds ratios are presented to show the relationship between HT monitoring and ED visits.
COPD
Veterans using HT had a greater proportion of ED visits compared to those who did not (41% vs. 27%, respectively; uOR: 1.90; 95%CI: 1.83–1.97) (Fig. 2). In the adjusted model, the odds of an ED visit were greater in the HT cohort compared to the non-HT cohort (aOR: 1.24; 95%CI: 1.18–1.31). Sensitivity analyses demonstrated overall similar results in most sub-groups (Supplemental Figure 9).
DM
Veterans who used HT had a greater proportion of ED visits compared to those who did not (26 vs. 22%, respectively; uOR: 1.24; 95%CI: 1.21–1.27) (Fig. 2). In the adjusted model, this relationship was attenuated (aOR: 1.03; 95%CI: 1.00–1.06). Visits were more likely to be in VHA EDs (aOR: 1.59; 95%CI: 1.47–1.71) (Supplemental Figure 10).
All-Cause Mortality
CHF
Veterans who used HT had a higher unadjusted 1-year all-cause mortality compared to those who did not (16% vs. 12%, respectively; uOR: 1.45, 95%CI: 1.40–1.50) (Fig. 3), but in the adjusted model, HT was associated with lower all-cause mortality (aOR 0.70; 95%CI: 0.67–0.73). Sensitivity analyses were similar (Supplemental Figure 11).
One-year all-cause mortality of matched cohort by chronic health condition and telehealth status. Each bar shows the proportion of veterans with each chronic condition that died during the 1-year follow-up (left panel). Gray bars show the proportion of veterans that did not use any home telehealth (HT) monitoring that died during the year, and black bars show the proportion of veterans who had HT monitoring that died during the year. Veterans using both clinical video telehealth and home telemonitoring in any given year were excluded from the sample. In the second panel (right), the unadjusted (gray) and adjusted (black) odds ratios are presented to show the relationship between HT monitoring and all-cause mortality.
COPD
Veterans who used HT had higher 1-year all-cause mortality compared to those who did not (17% vs. 8%, respectively; uOR: 2.50, 95%CI: 2.37–2.63) (Fig. 3), though this effect was attenuated in the multivariable model (aOR: 1.08; 95%CI: 1.08–1.16). Sensitivity analyses demonstrated similar results in all sub-groups (Supplemental Figure 12).
DM
Veterans who used HT had a higher unadjusted 1-year all-cause mortality compared to those who did not (7% vs. 5%, respectively; uOR: 1.49, 95%CI: 1.43–1.55) (Fig. 3), but similar to our CHF cohort, the adjusted model showed lower all-cause mortality in the HT group (aOR: 0.79; 95%CI: 0.75–0.83). Sensitivity analyses demonstrated similar results (Supplemental Figure 13).
DISCUSSION
In this study, we assessed the association between the use of the HT program within the VHA for patients with CHF, COPD, or DM and 1-year healthcare utilization and mortality. As an early adopter of telehealth modalities, the VHA presents a unique opportunity to evaluate the effect of a HT program on a national scale across medical conditions. Additionally, we examined sub-groups for potential variability that could be useful to target the intervention to patients most likely to benefit. In aggregate, we observed that HT patients with CHF and DM had increased ED visits, no change in hospitalizations, and lower all-cause mortality, while those with COPD had both higher healthcare utilization and all-cause mortality. We also showed that increased utilization in the HT group was mainly within VHA facilities, which has important economic implications for the VHA. These findings are relevant, because they suggest that HT for chronic disease management may function primarily as an effective early warning system, which might not reduce healthcare utilization, but is associated with a significant reduction in reduced mortality for 2 of the 3 chronic conditions assessed.
Our findings were consistent with anticipated outcomes of an HT program, which are to improve access to care and clinical outcomes.30 Our findings contradict some HT studies both within the VHA and non-VHA settings. In a recent meta-analysis of post-discharge virtual care across chronic diseases, virtual visits as part of a transitional care program were shown to reduce both mortality and hospital admissions after admission for CHF but not COPD, but no significant effect was observed in ED visits.31 The post-discharge interventions included in this systematic review were different from chronic disease HT, but similarly, used virtual care to supplement home-based chronic disease management. The meta-analysis included randomized trials and small cohort studies. Our study of a large, national cohort may better represent the effects of a mature intervention in an integrated network.
One significant difference between our work and prior telehealth studies is that we measured the effect of a mature VA HT intervention at scale. Health interventions that achieve maturity may achieve different results than pilot programs because they may lose fidelity to the original intervention, enroll different types of patients, function differently in a different context, or have different incentives outside the context of a clinical study. A 2006 study of an early application of HT for DM in the VHA showed decreased hospital admissions, but their cohort (n = 391) had a baseline admission risk almost twice our sample—suggesting that there may be heterogeneous treatment effects.32 A 2012 study of VHA HT for COPD also showed reduced healthcare utilization, but the study was limited only to those with the most frequent baseline annual COPD exacerbations—only 33% of the total number of COPD patients enrolled in the HT program in the participating center.33 Our study included the entire range of baseline severity of illness, and our results were more modest than those observed in studies of more severe baseline illness.
Some of the effects of HT could represent specific features of the HT intervention. In a recent report from a CHF HT program in France, an intensive educational and monitoring program was associated with fewer hospitalizations and decreased mortality, but a daily educational intervention was an important part of the program and the effect was greatest in those participants who engaged most with the telemonitoring service.34 In contrast, Hofer et al.35 reported the results of a HT program for COPD in Germany, which yielded similar results to our findings, increased healthcare utilization and more outpatient care and prescriptions.
We are encouraged by the association between HT and survival in CHF and DM. Importantly, HT was used for patients at high risk of poor short-term outcomes (16% 1-year mortality for CHF, 17% for COPD, and 7% for DM). Not surprisingly, those patients who required the most care were more likely to be enrolled in HT. The fact that HT was associated with increased ED visits with no difference in hospital admissions suggests that it was functioning partly as a surveillance system—identifying patients most likely to benefit from acute intervention. This relationship also increases confidence in our findings because, while residual confounding in our observational cohort could explain increased healthcare utilization, we would not expect residual confounding to explain improved survival. We did not measure the clinical interventions associated with HT, but future work could examine the frequency and timing of medication changes, dietary recommendations, or other clinician-directed care in the HT group. Early warning systems are most effective when decompensation can be reversed by specific interventions, such as those used for CHF and DM.
A finding relevant to any integrated health system is the extent to which HT enrollment was associated with receiving ED and inpatient care within the VHA system. Perhaps because of the early warning surveillance function of the HT intervention, veterans who required ED visits or hospital admissions were more likely to be treated in a VHA facility. This effect could be due to early identification of decompensated disease, when patients could still get to a VHA facility, or it could be because HT staff directly arranged for VHA care. As in many integrated health systems, though, this effect aligns closely with a strategic priority to keep VHA-funded care within the VHA system for improved efficiency and lower cost.36.
Another key observation was that the effect of HT varied by the chronic disease studied. While increased ED utilization and decreased mortality were consistent with similar effect sizes in CHF and DM, this was not the case for COPD. Conflicting evidence in HT use for COPD was previously identified in a recent review, which found low to very low quality of evidence across all outcomes. A 2019 meta-analysis of 27 studies demonstrated reductions in ED visits and hospitalizations overall with integrated HT, but this impact was significantly attenuated for COPD. These findings may not be unexpected because COPD is often treated with more stable medication regimens than CHF or DM and the need for short-term medication management may not require the technical fidelity of HT. Some elements of the outpatient management of patients with lower respiratory tract disease may be evolving as clinicians are increasingly comfortable with telehealth-enabled home management.37,38.
Our study has several limitations. First, our observational design using administrative data could be subject to residual confounding and data inconsistencies. We carefully selected parameters from the electronic health record that are likely to be accurately recorded, we verified mortality data with the National Death Index, and we used robust matching and regression adjustment in our analysis, but residual confounding may remain. The second limitation is that we are not able to accurately describe the mechanisms of telehealth interventions. For HT especially, the value of the intervention is knowing how education, medication changes, medication adherence, and other clinical interventions are applied in response to the data collected, but we do not have these interventions described in our data. Third, our data do not contain a measure of HT “dose,” which is the frequency of engagement with the HT service. We classified all patients accessing the intervention as an “exposed” patient, so any patients who stopped using the service were classified as being affected by the service. This approach more likely reflects actual behavior in a mature telehealth network, but we are not able to report treatment retention. Finally, our study was conducted in the VHA, and VHA-specific elements of care delivery and health system organization may differ substantially from non-VHA care so that the effect of telehealth may differ.
In conclusion, the initiation of HT is associated with increased ED visits, no reduction in hospital admissions, and decreased mortality in CHF and DM. HT use was also associated with healthcare being provided more in VHA facilities. Future work will focus on the specific interventions implemented using HT, developing care pathways that can reduce hospitalization and ED utilization, and better ways of integrating home care, telehealth, and outpatient management to optimize outcomes of chronic disease.
References
Buttorff C, Rudder T, Bauman M. Multiple Chronic Conditions in the United States. Santa Monica, CA. 2017. https://www.rand.org/content/dam/rand/pubs/tools/TL200/TL221/RAND_TL221.pdf. Accessed December 7, 2022.
Agency for Healthcare Research and Quality. HCUP Fast Stats - Most Common Diagnoses for Inaptient Stays. Agency for Healthcare Research and Quality. 2018. https://www.hcup-us.ahrq.gov/faststats/NationalDiagnosesServlet. Accessed December 7, 2022 2022.
Agarwal MA, Fonarow GC, Ziaeian B. National Trends in Heart Failure Hospitalizations and Readmissions From 2010 to 2017. JAMA Cardiol. 2021;6(8):952-6. doi:https://doi.org/10.1001/jamacardio.2020.7472
Shah T, Press VG, Huisingh-Scheetz M, White SR. COPD Readmissions: Addressing COPD in the Era of Value-based Health Care. Chest. 2016;150(4):916-26. doi:https://doi.org/10.1016/j.chest.2016.05.002
Rubin DJ. Correction to: Hospital Readmission of Patients with Diabetes. Curr Diabet Rep. 2018;18(4):21. doi:https://doi.org/10.1007/s11892-018-0989-1
Lee KK, Yang J, Hernandez AF, Steimle AE, Go AS. Post-discharge Follow-up Characteristics Associated With 30-Day Readmission After Heart Failure Hospitalization. Med Care. 2016;54(4):365-72. doi:https://doi.org/10.1097/mlr.0000000000000492
Wan TTH, Terry A, Cobb E, McKee B, Tregerman R, Barbaro SDS. Strategies to Modify the Risk of Heart Failure Readmission: A Systematic Review and Meta-Analysis. Health Serv Res Manag Epidemiol. 2017;4:2333392817701050-. doi:https://doi.org/10.1177/2333392817701050
Manemann SM, Sauver JS, Henning‐Smith C, Rutten LJF, Chamberlain AM, Fabbri M, et al. Rurality, Death, and Healthcare Utilization in Heart Failure in the Community. J Am Heart Assoc. 2021;10(4):e018026. doi:doi:https://doi.org/10.1161/JAHA.120.018026
Totten AM, Hansen RN, Wagner J, Stillman L, Ivlev I, Davis-O’Reilly C, et al. AHRQ Comparative Effectiveness Reviews. Telehealth for Acute and Chronic Care Consultations. Rockville (MD): Agency for Healthcare Research and Quality (US); 2019.
Salisbury C, Thomas C, Cathain A, Rogers A, Pope C, Yardley L, et al. TElehealth in CHronic disease: mixed-methods study to develop the TECH conceptual model for intervention design and evaluation. BMJ Open. 2015;5(2):e006448. doi:https://doi.org/10.1136/bmjopen-2014-006448
Heppner S, Mohr NM, Carter KD, Ullrich F, Merchant KAS, Ward MM. HRSA's evidence-based tele-emergency network grant program: Multi-site prospective cohort analysis across six rural emergency department telemedicine networks. PLoS One. 2021;16(1):e0243211. doi:https://doi.org/10.1371/journal.pone.0243211
Koehler F, Koehler K, Deckwart O, Prescher S, Wegscheider K, Kirwan BA, et al. Efficacy of telemedical interventional management in patients with heart failure (TIM-HF2): a randomised, controlled, parallel-group, unmasked trial. Lancet. 2018;392(10152):1047-57. doi:https://doi.org/10.1016/s0140-6736(18)31880-4
Darkins A. The growth of telehealth services in the Veterans Health Administration between 1994 and 2014: a study in the diffusion of innovation. Telemed J E Health. 2014;20(9):761-8. doi:https://doi.org/10.1089/tmj.2014.0143
Darkins A, Ryan P, Kobb R, Foster L, Edmonson E, Wakefield B, et al. Care Coordination/Home Telehealth: the systematic implementation of health informatics, home telehealth, and disease management to support the care of veteran patients with chronic conditions. Telemed J E Health. 2008;14(10):1118-26. doi:https://doi.org/10.1089/tmj.2008.0021
McMillian G. Telehealth Improves Heart Care for Veterans. VAntage Point - U.S. Department of Veterans Affairs, Washington, DC. 2021. https://blogs.va.gov/VAntage/96471/telehealth-improves-heart-care-for-veterans/. Accessed 2/22/2022.
Messina W. Decreasing Congestive Heart Failure Readmission Rates Within 30 Days at the Tampa VA. Nurs Adm Q. 2016;40(2):146-52. doi:https://doi.org/10.1097/naq.0000000000000154
Srivastava A, Do J-M, Sales VL, Ly S, Joseph J. Impact of patient-centred home telehealth programme on outcomes in heart failure. Journal of Telemedicine and Telecare. 2018;25(7):425-30. doi:https://doi.org/10.1177/1357633X18775852
Darkins A, Kendall S, Edmonson E, Young M, Stressel P. Reduced cost and mortality using home telehealth to promote self-management of complex chronic conditions: a retrospective matched cohort study of 4,999 veteran patients. Telemed J E Health. 2015;21(1):70-6. doi:https://doi.org/10.1089/tmj.2014.0067
Quan H, Li B, Couris CM, Fushimi K, Graham P, Hider P, et al. Updating and Validating the Charlson Comorbidity Index and Score for Risk Adjustment in Hospital Discharge Abstracts Using Data From 6 Countries. American Journal of Epidemiology. 2011;173(6):676-82. doi:https://doi.org/10.1093/aje/kwq433
Wang L, Porter B, Maynard C, Evans G, Bryson C, Sun H, et al. Predicting risk of hospitalization or death among patients receiving primary care in the Veterans Health Administration. Med Care. 2013;51(4):368-73. doi:https://doi.org/10.1097/MLR.0b013e31827da95a
Fihn SD, Francis J, Clancy C, Nielson C, Nelson K, Rumsfeld J, et al. Insights from advanced analytics at the Veterans Health Administration. Health Aff (Millwood). 2014;33(7):1203-11. doi:https://doi.org/10.1377/hlthaff.2014.0054
Statistics NCfH. National Death Index. Centers for Disease Control and Prevention. 2022. https://www.cdc.gov/nchs/ndi/index.htm. Accessed 2.22.2022.
von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370(9596):1453-7. doi:https://doi.org/10.1016/s0140-6736(07)61602-x
Chumbler NR, Neugaard B, Kobb R, Ryan P, Qin H, Joo Y. Evaluation of a Care Coordination/Home-Telehealth Program for Veterans with Diabetes: Health Services Utilization and Health-Related Quality of Life. Eval Health Professions. 2005;28(4):464-78. doi:https://doi.org/10.1177/0163278705281079
Kobb R, Hoffman N, Lodge R, Kline S. Enhancing elder chronic care through technology and care coordination: report from a pilot. Telemed J E Health. 2003;9(2):189-95. doi:https://doi.org/10.1089/153056203766437525
Meyer M, Kobb R, Ryan P. Virtually Healthy: Chronic Disease Management in the Home. Dis Manag. 2002;5(2):87-94. doi:https://doi.org/10.1089/109350702320229186
U.S. Department of Veterans Affairs. VA Telehealth Services. 2022. https://telehealth.va.gov/type/home. Accessed June 3, 2022.
U.S. Department of Veterans Affairs. Rural Telehealth. 2020. https://www.ruralhealth.va.gov/docs/ORH_Rural-Telehealth-InfoSheet_2020_FINAL_508.pdf. Accessed 10/24/2022.
U.S. Department of Veterans Affairs. Surveillance of All Veteran Suicides. 2022. https://www.mirecc.va.gov/suicideprevention/Data/data_index.asp#:~:text=VA%2FDoD%20Mortality%20Data%20Repository%20(MDR)&text=The%20MDR%20provides%20the%20most,VA%20and%20DoD%20administrative%20records. Accessed 10/24/2022.
Department of Veteran Affairs. Fact Sheet. VA Telehealth Services. https://www.va.gov/COMMUNITYCARE/docs/news/VA_Telehealth_Services.pdf. Accessed July 26, 2022.
Chauhan U, McAlister FA. Comparison of Mortality and Hospital Readmissions Among Patients Receiving Virtual Ward Transitional Care vs Usual Postdischarge Care: A Systematic Review and Meta-analysis. JAMA Netw Open. 2022;5(6):e2219113. doi:https://doi.org/10.1001/jamanetworkopen.2022.19113
Barnett TE, Chumbler NR, Vogel WB, Beyth RJ, Qin H, Kobb R. The effectiveness of a care coordination home telehealth program for veterans with diabetes mellitus: a 2-year follow-up. Am J Manag Care. 2006;12(8):467-74.
Alrajab S, Smith TR, Owens M, Areno JP, Caldito G. A home telemonitoring program reduced exacerbation and healthcare utilization rates in COPD patients with frequent exacerbations. Telemed J E Health. 2012;18(10):772-6. doi:https://doi.org/10.1089/tmj.2012.0005
Sabatier R, Legallois D, Jodar M, Courouve L, Donio V, Boudevin F, et al. Impact of patient engagement in a French telemonitoring programme for heart failure on hospitalization and mortality. ESC Heart Fail. 2022. doi:https://doi.org/10.1002/ehf2.13978
Hofer F, Schreyögg J, Stargardt T. Effectiveness of a home telemonitoring program for patients with chronic obstructive pulmonary disease in Germany: Evidence from the first three years. PLoS One. 2022;17(5):e0267952. doi:https://doi.org/10.1371/journal.pone.0267952
Department of Veteran Affairs. Department of Veterans Affairs Fiscal Years 2022–28 Strategic Plan. 2022. https://www.va.gov/oei/docs/va-strategic-plan-2022-2028.pdf. Accessed November 21, 2022.
Bryant AD, Robinson TJ, Gutierrez-Perez JT, Manning BL, Glenn K, Imborek KL, et al. Outcomes of a home telemonitoring program for SARS-CoV-2 viral infection at a large academic medical center. J Telemed Telecare. 2022:1357633x221086067. https://doi.org/10.1177/1357633x221086067
Dirikgil E, Roos R, Groeneveld GH, Heringhaus C, Silven AV, Petrus AHJ, et al. Home monitoring reduced short stay admissions in suspected COVID-19 patients: COVID-box project. Eur Respir J. 2021;58(2). doi:https://doi.org/10.1183/13993003.00636-2021
Acknowledgements
The authors would like to acknowledge Koreen Luerkens, RN, from the Iowa City VHA Home Telehealth Program, for her guidance on the telehealth processes within the VHA, and Paul Casella, MFA, and Mariah Temple, BA, for their editorial assistance.
Funding
This study was supported by funding from the Office for the Advancement of Telehealth, Health Resources and Services Administration (U3G RH40003). Additionally, this material is based upon work supported (or supported in part) by the Department of Veterans Affairs, Veterans Health Administration, Office of Rural Health, Veterans Rural Health Resource Center- Iowa City (Award #16020). The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the United States government.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors report that they have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mohr, N.M., Vakkalanka, J.P., Holcombe, A. et al. Effect of Chronic Disease Home Telehealth Monitoring in the Veterans Health Administration on Healthcare Utilization and Mortality. J GEN INTERN MED 38, 3313–3320 (2023). https://doi.org/10.1007/s11606-023-08220-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11606-023-08220-5