Abstract
Introduction
International guidelines provide heterogenous guidance on use of corticosteroids for community-acquired pneumonia (CAP).
Methods
We performed a systematic review of randomized controlled trials examining corticosteroids in hospitalized adult patients with suspected or probable CAP. We performed a pairwise and dose-response meta-analysis using the restricted maximum likelihood (REML) heterogeneity estimator. We assessed the certainty of the evidence using GRADE methodology and the credibility of subgroups using the ICEMAN tool.
Results
We identified 18 eligible studies that included 4661 patients. Corticosteroids probably reduce mortality in more severe CAP (RR 0.62 [95% CI 0.45 to 0.85]; moderate certainty) with possibly no effect in less severe CAP (RR 1.08 [95% CI 0.83 to 1.42]; low certainty). We found a non-linear dose-response relationship between corticosteroids and mortality, suggesting an optimal dose of approximately 6 mg of dexamethasone (or equivalent) for a duration of therapy of 7 days (RR 0.44 [95% 0.30 to 0.66]). Corticosteroids probably reduce the risk of requiring invasive mechanical ventilation (RR 0.56 [95% CI 0.42 to 74] and probably reduce intensive care unit (ICU) admission (RR 0.65 [95% CI 0.43 to 0.97]) (both moderate certainty). Corticosteroids may reduce the duration of hospitalization and ICU stay (both low certainty). Corticosteroids may increase the risk of hyperglycemia (RR 1.76 [95% CI 1.46 to 2.14]) (low certainty).
Conclusion
Moderate certainty evidence indicates that corticosteroids reduce mortality in patients with more severe CAP, the need for invasive mechanical ventilation, and ICU admission.
Similar content being viewed by others
INTRODUCTION
Several randomized controlled trials (RCTs) examining the role of corticosteroids in hospitalized adult patients with severe pneumonia demonstrate benefit in improving survival. This includes patients with coronavirus disease 2019 (COVID-19), and those who develop acute respiratory distress syndrome (ARDS).1,2,3 Nevertheless, the adjunctive use of corticosteroids for community-acquired pneumonia (CAP) remains variable and provider dependent. International societal guidelines are heterogeneous; many recommend against the routine use of corticosteroids in patients with CAP, except in cases of refractory septic shock.4,5,6 The justification against the routine use of corticosteroids includes low certainty data owing to both statistical and clinical heterogeneity, and ongoing issues with imprecision of pooled estimates. Previous analyses have been limited by few and underpowered studies, as well as a lack of analysis of optimal dosing.7
With the publication of a few large recent RCTs including the recent ESCAPe and CAPE COD trials examining this question, and with the objective of carefully evaluating key components of corticosteroid regimes, including dose and duration of therapy, we aimed to perform an updated systematic review and pairwise and dose-response meta-analysis of RCTs examining the role of corticosteroids in patients hospitalized with bacterial CAP.
METHODS
We generated the study protocol using the PRISMA-P guidelines and registered it on Open Science Framework: https://osf.io/nqm28.
Search Strategy
With the help of an experienced medical librarian, we developed a comprehensive search strategy (eTables 1–4). The search strategy was based on the last major review which was first published in 2015, then updated in February 2020.2,7 We searched Embase, Medline, Cochrane Central Register of Controlled Trials (CENTRAL), and clinicaltrials.gov for eligible trials from February 29, 2020, to September 5, 2022. We also reviewed the results from clinicaltrials.gov for updated trial results monthly. We also reviewed previous systematic reviews addressing the topic to ensure no studies were missed.1,2,3 We did not use any language restrictions and included only primary source clinical trial data. We reviewed secondary analyses and post hoc analysis for subgroup data, as required.
Eligibility Criteria
We included all RCTs that randomized adult patients (≥18 years old) hospitalized with probable or suspected CAP to treatment with corticosteroids versus standard care or placebo. We defined CAP in keeping with individual trial definitions incorporating clinical, microbiological, and/or radiographic evidence of bacterial pneumonia. We included studies of alternative doses or types of corticosteroids for the dose-response meta-analysis. If patients were hospitalized, we included all severities of disease but planned an a priori subgroup analysis based on more severe vs less severe patients. We defined trials as more severe if 50% or more of the participants had severe pneumonia scores (pneumonia severity scores of IV or V, CURB65 scores of ≥3, CORB scores of ≥2, SMART-COP scores ≥4), or if ≥50% of patients were admitted to the intensive care unit (ICU) at the time of randomization. We excluded trials that enrolled predominately (≥80%) patients with Pneumocystis jirovecii pneumonia, inflammatory cases of pneumonia such as organizing pneumonia, chronic obstructive pulmonary disease (COPD), COVID-19 pneumonia, other viral cases of pneumonia, empyema, post-obstructive pneumonia, or ventilator-associated pneumonia.
STUDY SELECTION AND DATA EXTRACTION
We used COVIDENCE to screen eligible trials. Two reviewers (TP, DZ), following training and calibration exercises to ensure sufficient agreement, worked independently and in duplicate to screen titles and abstracts of search records and subsequently the full texts of records that were determined potentially eligible at the title and abstract screening stage. Reviewers resolved discrepancies by discussion or, when necessary, by third-party adjudication. Similarly, the two reviewers worked independently and in duplicate to extract data from eligible trials, and resolved discrepancies by discussion or, when necessary, by third-party adjudication (BR).
We collected data on trial characteristics (author, year published, trial registration, country of enrollment), patient characteristics (age, sex, comorbidities, C-reactive protein (CRP), white blood cell (WBC) count, proportion of patients on oxygen, and proportion of patients in ICU), intervention characteristics (type of corticosteroid, dose, duration), and outcomes of interest. Outcomes of interest included mortality, need for invasive mechanical ventilation (in those not requiring invasive mechanical ventilation at baseline), secondary infections (any type and severity), gastrointestinal (GI) bleeding (defined by study authors, any severity), ICU admission (in those not requiring ICU admission at baseline), hyperglycemia (requiring intervention), and duration of ICU and hospital stay. For all dichotomous outcomes, we collected data at the longest follow-up or closest to 90 days.
For dichotomous outcomes, we extracted the number of participants analyzed and the number of events in each arm. For continuous outcomes, we collected data on the point estimate of the mean and standard deviation. When studies reported other measures of variability other than standard deviation, we converted them to standard deviations using methods proposed by Hozo et al.8.
Risks of Bias
We rated the risk of bias at an outcome level. Two reviewers, working independently and in duplicate, assessed the risk of bias for individual RCTs using a revision of the Cochrane tool (RoB 2.0).9,10,11 We rated the risk of bias as either at (i) low risk of bias, (ii) probably low risk of bias, (iii) probably high risk of bias, or (iv) high risk of bias, across the following domains: bias arising from the randomization process; departures from the intended intervention; missing outcome data; measurement of the outcome; and selection of the reported results. We classified trials rated as definitely or probably low risk of bias across domains as low risk of bias overall. We resolved discrepancies by discussion and, when necessary, with adjudication by a third party. eTable 5 presents the risk of bias tool.
Statistical Methods
Pairwise Meta-Analysis
For all outcomes, we performed a random-effects meta-analysis with the restricted maximum likelihood (REML) heterogeneity estimator.12 We summarized the effects of interventions using relative risk (RR) for dichotomous outcomes and mean differences (MD) for continuous outcomes, both with associated 95% confidence intervals (CIs). To facilitate the interpretation of dichotomous outcomes, we also calculated absolute risk differences (RD) per 1000 patients and corresponding 95% confidence intervals.13,14,15 We calculated the baseline risk using the median risk in the standard care arms of the included trials.
One trial was a cluster randomized trial and therefore we calculated the associated effective sample size using the design effect as described in Cochrane guidance.16
Dose-Response Analysis
For mortality and adverse events found to be statistically significant, we performed an additional dose-response meta-analysis. For the dose-response analysis, we conducted a random-effects dose-response meta-analysis using the restricted maximum likelihood (REML) heterogeneity estimator and methods proposed by Greenland and colleagues17,18 using a one-stage approach.19 Dose-response meta-analysis estimates the association between doses of an exposure and the relative risk or mean difference of an outcome. We analyzed the cumulative dose of corticosteroids administered during the trial by multiplying the administered dose by the duration (i.e., dexamethasone 6 mg/day for 10 days = 60 mg total dose).
To address whether results are driven by cumulative exposure to corticosteroids or the duration of exposure, we performed a prespecified random-effects meta-regression and included the duration of treatment with corticosteroids as a moderator. We anticipated that if the duration of exposure rather than cumulative exposure was important, we would see larger effects in the analysis of duration rather than cumulative dose.
We used the following corticosteroid conversions: 1 mg of dexamethasone = 26.7 mg of hydrocortisone = 5.3 mg of methylprednisolone/prednisolone= 6.7 mg of prednisone.20,21,22 We divided the total dose by the median number of days across trials. To ensure no differences based on molecule, we performed meta-regression using molecule as a moderator.
For analyses with five or more studies, we assessed for non-linearity by using restricted cubic splines with knots at 10%, 50%, and 90% percentiles and a Wald-type test.23 Restricted cubic splines accommodate non-linear relationships by splitting the independent variable (i.e., dose) at “knots” and fitting separate curves between knots. For analyses in which we observed statistically significant non-linear associations, we present results from the non-linear model.
We assessed model fit by calculating deviance, adjusted and unadjusted coefficients of determination, and by decorrelated residuals-versus-exposure plot.24 We assessed heterogeneity by inspection of forest plots, the I2 statistic, and the chi-squared test. We considered heterogeneity ranging from 0 to 40% as potentially unimportant, 30 to 60% as moderate heterogeneity, 50 to 90% as substantial heterogeneity, and 75 to 100% as critical heterogeneity.16 For outcomes with 10 or more studies, we tested for publication bias or small study effects using both visual inspection of funnel plots and Egger’s test.25
For mortality, we conducted trial sequential analysis (TSA) using a random-effects model.26 For the TSA, we used a statistical significance level of 5%, a power of 80%, and a relative risk reduction of 38%. We used a model variance-based heterogeneity correction TSA performed using Trial Sequential Analysis v.0.9.5.10 beta (Copenhagen Trial Unit, Centre for Clinical Intervention Research, Rigshospitalet, Copenhagen, Denmark, www. ctu.dk/tsa).
We performed all analyses using the dosresmeta and meta packages in R (version 4.03, R Foundation for Statistical Computing).27,28 We used STATA v.17 for pairwise analyses.
A Priori Subgroup Analysis
For trials that reported on patients with less severe and more severe diseases, we extracted in-study subgroup data for outcomes of interest. When in-study subgroups were not reported, we decided on trial-level severity based on whether 50% or more of patients were categorized as severe using a validated severity score, whether patients were predominately ICU patients, or if they required vasopressors.
We performed subgroup analysis based on (1) severe CAP versus non-severe CAP, hypothesizing that those with severe disease may benefit more from corticosteroids due to a more dysregulated inflammatory response, (2) high risk of bias versus low risk of bias, hypothesizing that higher risk of bias trials would show a larger effect than the lower risk of bias trials, (3) corticosteroid type, dexamethasone versus hydrocortisone versus methylprednisolone versus prednisone/prednisolone, hypothesizing no effect, and (4) the duration of therapy (including taper), comparing trials treating for <7 days versus ≥7 days, with the hypothesis that longer duration would be more effective than shorter duration.
We assessed the credibility of statistically significant subgroups using the Instrument for Assessing the Credibility of Effect Modification Analyses (ICEMAN) tool.29
Certainty of the Evidence
For all outcomes, reviewers, working independently and in duplicate, assessed the certainty of the evidence using the GRADE approach.30,31 We judged the certainty for each outcome as high, moderate, low, or very low, based on considerations of risk of bias, inconsistency, indirectness, imprecision, and publication bias.
To make judgments regarding imprecision, we used a minimally contextualized approach, which considers only whether confidence intervals include a minimally important effect and does not consider the magnitude of plausible effects, captured by confidence intervals.32 We used a minimally important difference (MID) based on consensus of the authors. For mortality, we chose an MID of 1%, and for all other dichotomous outcomes, we chose a 2% MID. For the duration of hospitalization, ICU stay, and ventilator-free days, we chose 1 day as MID. Using updated GRADE guidance, we rated imprecision using the confidence interval method, whereby if the confidence intervals included the MID in one direction, we rated down once; if in two directions, we rated down twice.33
We described our results using guidance from the GRADE Working Group, based on the certainty of evidence and the magnitude of the effect (e.g., corticosteroids reduce mortality [high certainty], corticosteroids probably reduce mortality [moderate certainty], corticosteroids may reduce mortality [low certainty], and the effect of corticosteroids on mortality is very uncertain [very low certainty]).31
RESULTS
Search Results
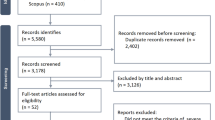
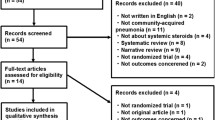
The search yielded 2666 unique citations. We identified a total of 18 eligible studies that included 4661 patients.34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50 All trials were published in peer-reviewed journals, and all in English except for one article which was published in Mandarin (Chinese)35. Figure 1 presents the PRISMA flow diagram.
Trial and Participant Characteristics
Table 1 presents trial and participant characteristics. Trials recruited adult patients with a median age of 64.35 years old (interquartile range [IQR] 69.4 to 57.9). Most patients were male (70.5%) and approximately 1/3 of patients were admitted to the ICU at the time of randomization. Bacterial culture was identified in 1622 (34.8%) and 205 (4.4%) had positive viral culture or PCR (including influenza). Four-hundred and sixty-seven (10.0%) were receiving invasive mechanical ventilation, and 214 patients (4.6%) required vasopressors at randomization.
We classified ten trials as studying more severe diseases,35,36,37,38,43,45,46,48,50 and eight as studying non-severe diseases34,39,40,41,42,44,47,49. Table 1 presents more details on how we categorized severity.
Of the trials that reported a pneumonia severity index (PSI), scores ranged from 89.5 to 123.9. Trials included patients who were classified with class V PSI scores ranging from 9.7 to 40%, indicating significant heterogeneity in severity across trials. The median CRP across included trials was 217 mg/L (IQR 126.9 to 254.3).
Seven trials (1178 patients) examined hydrocortisone,36,37,38,43,48,49 four trials (807 patients) methylprednisolone,35,45,46,50 five trials (1971) prednisolone/prednisone,34,39,42,44,47 and two trials (705) examined dexamethasone.40,41 Seven trials reported on durations of less than 7 days and two trials longer than 7 days. The median treatment duration was 7 days (IQR 5 to 7).
The median total dose used across trials was 70.3 mg (IQR 26.05 to 77.25) of dexamethasone equivalent, divided between 7 days for a daily dose equivalent of approximately 10 mg of dexamethasone equivalent/day.
Risk of Bias
For mortality, seven trials (43.7%, 1194 patients) were at probable or high risk of bias due to issues with the randomization process,34,35,36,43,44,48,49 seven trials (43.7%) due to deviations from the intended interventions,34,35,36,43,44,48,49 two trials were at risk of bias due to missing data (12.5%),34,35 and four trials (25%) due to selective reporting of the results.34,35,44,48 eTable 6 presents more details on the risk of bias assessments for all outcomes.
Mortality
Seventeen trials (4567 patients) reported on mortality, with 443 deaths.34,35,36,37,38,40,41,42,43,44,45,46,47,48,49,50 For patients with more severe pneumonia, corticosteroids probably reduce mortality as compared to usual care (RR 0.62 [95% CI 0.45 to 0.85]; moderate certainty), with an absolute risk difference of 56 fewer deaths per 1000 patients [95% CI 81 to 22 fewer]. For patients with less severe pneumonia, corticosteroids may have no effect on mortality as compared to usual care (RR 1.08 [0.83 to 1.42]; low certainty), with an absolute risk difference of 6 more per 1000 (95% CI 13 fewer to 32 more). Figure 2 presents the forest plot and Table 2 presents the summary of findings.
We found a statistically significant (p=0.01) subgroup effect based on the severity of pneumonia and rated the credibility as moderate using the ICEMAN tool (eTable 7, eFigures 1-2). There was no subgroup effect based on the risk of bias, decade studied, or duration of corticosteroid use (eFigures 3-5). There was a statistically significant subgroup effect based on the type of corticosteroid in the subgroup analysis (p<0.001) and in meta-regression (p=0.001), with moderate/low credibility using ICEMAN. Figure 3 and eFigure 5-6 and eTable 7 present the results.
Forest plot for mortality based on corticosteroid type subgroup. The left column shows the individual studies included in the meta-analysis, the middle column represents the effect sizes, and the right column shows the individual relative risks and their weight in contributing to the overall estimates.
The TSA showed the required information size was not met (eFigure 7).
Dose Response
The dose-response meta-analysis demonstrated a non-linear dose-response relationship. We found that the highest impact on mortality was evident with relatively lower doses of dexamethasone or dexamethasone equivalents, with the optimal dosing being approximately 6 mg of dexamethasone daily for seven days (RR 0.45 [95% CI 0.0.32 to 0.68]). Relatively higher doses, above 11.5 mg of dexamethasone per day for 7 days, were associated with no effect or harm. Figure 4 and Table 3 present the dose-response curve data and eTable 8 presents goodness of fit statistics.
Invasive Mechanical Ventilation
Nine trials (2895 patients), with patients who did not require IMV at randomization, reported on the need for invasive mechanical ventilation, with 185 events.34,36,38,42,45,46,47,48 Corticosteroids probably reduce the need for invasive mechanical ventilation as compared to usual care (RR 0.56 [95% CI 0.42 to 0.74]; moderate certainty), with an absolute risk difference of 82 fewer events per 1000 [95% CI 21 to 48 fewer].
There was no subgroup effect for non-severe versus severe, risk of bias, or type of corticosteroid or duration of treatment (eFigures 8-11), and Table 2 presents the summary of findings.
Need for ICU Admission
Five trials (2227 patients), with patients who did not require ICU at randomization reported on the need for ICU admission, with 95 events.34,40,41,45,47 Corticosteroids probably reduce the need for ICU admission as compared to usual care (RR 0.65 [0.43 to 0.97]; moderate certainty) with an absolute risk difference of 18 fewer cases per 1000 [95% CI 2 to 29 fewer]. There was no subgroup difference for risk of bias, type of corticosteroid, or duration of treatment. eFigures 12-14 present the forest plots and Table 2 presents the summary of findings.
Duration of Hospitalization
Thirteen trials (3442 patients) reported on duration of hospitalization, with a mean duration of hospitalization of 12.8 days.34,35,36,38,39,40,41,42,43,45,46,47,50 Corticosteroids may reduce the duration of hospitalization as compared to usual care (MD 2.31 days fewer [95% CI 0.76 to 3.85 fewer]; low certainty). There was no subgroup effect for severity, type of corticosteroid, or duration of treatment. There was a statistically significant subgroup effect for risk of bias; we took this into account when rating the certainty of the evidence (eTable 7). eFigures 15-18 present the forest plots and Table 2 presents the summary of findings.
Duration of ICU Stay
Eleven trials (926 patients) reported on duration of ICU stay, with a mean duration of hospitalization of 9.9 days.36,38,40,43,45,46,47,48,50 Corticosteroids may reduce the duration of ICU stay (MD 2.1 days fewer [95% CI 0.50 to 3.61 days fewer]; low certainty). There was no subgroup effect for severity, risk of bias, type of corticosteroid, or duration of treatment (eFigures 19-23). Table 2 presents the summary of findings.
Adverse Events: Secondary Infections, Gastrointestinal Bleeding, and Hyperglycemia
Ten (2970 patients) trials reported on secondary infections,35,38,39,40,42,43,46,47,50 eleven trials (3362) trials reported on hyperglycemia,34,35,36,39,40,41,42,45,46,47,50 and eleven trials (3368 patients) reported on gastrointestinal bleeding.34,35,36,37,38,43,45,46,47,50 Corticosteroids may have no effect on the risk of secondary infections (RR 1.09 [95% CI 0.85 to 1.41]; low certainty) or gastrointestinal bleeding (RR 0.95 [95% CI 0.56 to 1.60]; low certainty) compared to usual care. Corticosteroids probably increase the risk of hyperglycemia when compared to usual care (RR 1.76 [95% CI 1.46 to 2.14]; moderate certainty) with an absolute risk difference of 58 more per 1000 (95% CI 35 more to 87 more).
Five trials reported on hyperglycemia requiring insulin,34,35,42,45,50 whereas four reported only on the incidence of hyperglycemia.36,39,40,41,46 However, there was no difference in the overall effect in the subgroup analysis (eFigure 36).
There was no subgroup effect for the type of corticosteroid, duration of treatment, or risk of bias for any of the adverse events. There was a statistically significant subgroup effect for severity and hyperglycemia, which we rated as low credibility. eFigures 24-36 present the forest plots and subgroups.
Dose Response
The dose-response meta-analysis for hyperglycemia demonstrated both a non-linear and linear dose-response relationship, although the non-linear model is very uncertain for doses above 8.5 mg of dexamethasone/7 days. Both the non-linear and linear models suggest increasing harm with higher doses of corticosteroids.eTables 9 and 10 and eFigures 38-9 present the goodness of fit statistic and dose-response curve for hyperglycemia. eFigures 40-443 and eTables 11-14 present our publication bias assessments.
DISCUSSION
Main Findings
We present a comprehensive analysis examining the use of corticosteroids in hospitalized adult patients with CAP. We carefully evaluate between- and within-study subgroups to provide clinicians and evidence users with a nuanced assessment of specific patient populations that may benefit from corticosteroids. We found that corticosteroids reduce mortality in patients with severe CAP. We also demonstrate a non-linear dose-response relationship with mortality, with an optimal dose regimen that is relatively lower than the average used across trials. We found that corticosteroids probably reduce the risk of receiving invasive mechanical ventilation, and the need for ICU admission.
We also show that corticosteroids may reduce the duration of hospitalization and ICU stay, without substantially increasing the risk of secondary infections or GI bleeding. However, the use of corticosteroids may increase the incidence of hyperglycemia, requiring therapy such as insulin initiation or dose escalation.
In Relation to Other Findings
There have been two recent trials which showed diverging results. The recently published ESCAPe trial demonstrated no difference in 60-day mortality with corticosteroids (adjusted odds ratio 0.90, 95% CI 0.57 to 1.40).50 The ESCAPe trial has other important considerations that may influence the internal and external validity of the findings including prolonged recruitment time, resulting in early trial termination.38 In contrast, the recent CAPE COD trial demonstrated a favorable 28-day and 90-day mortality in patients with severe pneumonia receiving a median duration of hydrocortisone for 6 days (RR 0.53 [95% CI 0.33 to 0.84]). This study also has its limitations, namely it was stopped early for benefit.
The trials are similar in their trial design, sample size, and relatively similar corticosteroid dosing. One potentially important difference is the choice of corticosteroids. We found a moderate/low subgroup difference for hydrocortisone as compared to other corticosteroids. In fact, all the positive trials in this population, including the recent CAPE COD trial, have investigated hydrocortisone over alternatives.
Although previous systematic reviews and meta-analyses have demonstrated similar findings, we believe that this study provides important contributions. Specifically, as compared to the most recent review addressing this topic,51 of which there are significant methodological limitations and the absence of the CAPE COD trial,52 we have specifically evaluated for subgroup effect based on the severity of CAP and assess the credibility of potential subgroup effect using the ICEMAN tool. This subgroup analysis demonstrates that the effects of corticosteroids on mortality are limited to those with severe disease. Second, we have carefully applied GRADE which has led to substantial differences in certainty of evidence compared to the most recent review, leading to more nuanced conclusions regarding the effect of corticosteroid across outcomes. Third, to our knowledge, we are the first group to present a dose-response analysis informing the optimal dosing of corticosteroids in patients with severe CAP. We believe this is especially helpful given that previous guidelines often cite issues with uncertainty regarding optimal dose regimens. We converted the corticosteroid regimen to dexamethasone equivalents; however, previously there was currently no definitive evidence for use of one corticosteroid over another, although our analysis may provide some evidence for hydrocortisone over alternatives. The corticosteroid type subgroup showed a statistical effect on the mortality primary outcome, but this finding was rated as moderate/low credibility, and there was no effect for any of the other outcomes. Fourth, we provide increased precision by including newly available RCTs such as the ESCAPe and CAPE COD trial which randomized a combined 1379 patients with severe CAP. Incorporating this new data, we are now able to demonstrate with improved certainty the benefit of corticosteroids for improving survival in patients with severe CAP.
The COVID-19 pandemic has increased the attention on this intervention given the efficacy of corticosteroids in patients with severe-to-critical COVID-19 pneumonia. The definitive trial demonstrating benefit in COVID-19 pneumonia came from a large platform trial (RECOVERY), which randomized over 6000 patients. In contrast, the totality of the evidence evaluating corticosteroids in CAP includes less than 5000 patients, highlighting the ongoing uncertainty of corticosteroids for this indication. Although COVID-19 and bacterial CAP are unique disease processes, the underlying pathophysiology driven by lung inflammation is likely similar and the consistent beneficial effect of corticosteroids in downregulating cytokine production and inhibition of neutrophil and macrophage migration to the lungs between conditions has good biological rationale. Furthermore, there is an established benefit for corticosteroids in ARDS, a disease state with increasing inflammatory dysregulation.
Strengths and Limitations
Strengths of our review include the addition of recent trials that provide important in-study subgroups evaluating the impact of disease severity, allowing for a better understanding of the appropriate population that is most likely to benefit from corticosteroids. Furthermore, the authorship group includes experts in CAP and meta-analysis. We also performed careful subgroup analysis and assessed the credibility of the analysis using the validated ICEMAN tool.
Limitations include the paucity of trials evaluating and reporting within-study subgroups. Most trials did not report in-study subgroups based on severity and severity was heterogeneously defined across the trials. Furthermore, we still have only low certainty evidence for several outcomes including adverse events as well as duration of ICU and hospitalization.
It is unclear whether the effect of corticosteroids is consistent across different causes of bacterial pneumonia (i.e., streptococcus pneumonia vs other causes). Future studies should examine microbiologic subgroups.
We included a small number of patients who had confirmed viral pneumonia. The effect of corticosteroids on viral pneumonia is unclear and our conclusions are limited to bacterial pneumonia. Future studies are needed to examine the effect of corticosteroids in patients with viral pneumonia.
We chose an optimal duration of corticosteroid administration based on the median duration included in randomized trials. There may be conditions such as secondary organizing pneumonia, or severe ARDS, which warrant longer durations than 7 days. Unfortunately, these patients were not included in these RCTs and therefore we are unable to analyze them. Furthermore, to minimize adverse events from corticosteroids, future studies should investigate optimal duration (e.g., 5 versus 7 days versus longer).
Implications and Future Directions
Clinical practice guidelines differ in their recommendations for the use of corticosteroids in severe CAP. The 2017 Society of Critical Care Medicine/European Society of Intensive Care Medicine Guidelines for the Diagnosis and Management of Critical Illness-Related Corticosteroid Insufficiency in Critically Ill Patients conditionally recommends the use of corticosteroids in patients hospitalized with CAP.53 However, the American Thoracic Society (ATS), the Infectious Disease Society of America (IDSA), and the British Thoracic Society (BTS) recommended against the routine use of corticosteroids in CAP.54 Concerns include a lack of clarity around which populations would benefit from corticosteroids, quality of the RCTs, safety, and consistency of existing meta-analyses. This review addresses some of these concerns and may provide further justification for using corticosteroids in this population, especially those with severe disease. Also, we offer clinicians guidance on optimal dosing for corticosteroids. These results should inform updates of clinical practice guidelines and may help decrease variation in practice recommendations. Further research is needed to better ascertain if certain types of patients with CAP are more likely to benefit from corticosteroids than others, based on specific severity criteria, biomarkers, or other considerations.
CONCLUSION
We show that corticosteroids reduce mortality in patients with severe pneumonia and probably reduce the need for invasive mechanical ventilation and the need for ICU admission. Corticosteroids probably increase the risk of hyperglycemia without an effect on the risk of secondary infections or GI bleeding.
Data Availability:
Data will be available on Open Science Framework upon publication.
References
Chaudhuri D, Sasaki K, Karkar A, Sharif S, Lewis K, Mammen MJ, et al. Corticosteroids in COVID-19 and non-COVID-19 ARDS: a systematic review and meta-analysis. Intensive Care Med. 2021;47(5):521-37.
Ye Z, Wang Y, Colunga-Lozano LE, Prasad M, Tangamornsuksan W, Rochwerg B, et al. Efficacy and safety of corticosteroids in COVID-19 based on evidence for COVID-19, other coronavirus infections, influenza, community-acquired pneumonia and acute respiratory distress syndrome: a systematic review and meta-analysis. Cmaj. 2020;192(27):E756-e67.
Stern A, Skalsky K, Avni T, Carrara E, Leibovici L, Paul M. Corticosteroids for pneumonia. Cochrane Database Syst Rev. 2017;12(12):Cd007720
Metlay JP, Waterer GW, Long AC, Anzueto A, Brozek J, Crothers K, et al. Diagnosis and Treatment of Adults with Community-acquired Pneumonia. An Official Clinical Practice Guideline of the American Thoracic Society and Infectious Diseases Society of America. American Journal of Respiratory and Critical Care Medicine. 2019;200(7):e45-e67.
Lim WS, Baudouin SV, George RC, Hill AT, Jamieson C, Le Jeune I, et al. BTS guidelines for the management of community acquired pneumonia in adults: update 2009. Thorax. 2009;64 Suppl 3:iii1-55.
Boyles TH, Brink A, Calligaro GL, Cohen C, Dheda K, Maartens G, et al. South African guideline for the management of community-acquired pneumonia in adults. J Thorac Dis. 2017;9(6):1469-502.
Siemieniuk RA, Meade MO, Alonso-Coello P, Briel M, Evaniew N, Prasad M, et al. Corticosteroid Therapy for Patients Hospitalized With Community-Acquired Pneumonia: A Systematic Review and Meta-analysis. Ann Intern Med. 2015;163(7):519-28.
Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:13.
Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. Bmj. 2019;366:l4898.
Gordon Guyatt JB. Methods Commentary: Risk of Bias in Randomized Trials 1. 2021.
Pitre T, Mah J, Helmeczi W, Khalid M, Cui S, Zhang M, et al. Medical treatments for idiopathic pulmonary fibrosis: a systematic review and network meta-analysis. Thorax. 2022.
Langan D. Assessing Heterogeneity in Random-Effects Meta-analysis. Methods Mol Biol. 2022;2345:67-89.
Forrow L, Taylor WC, Arnold RM. Absolutely relative: how research results are summarized can affect treatment decisions. Am J Med. 1992;92(2):121-4.
Naylor CD, Chen E, Strauss B. Measured enthusiasm: does the method of reporting trial results alter perceptions of therapeutic effectiveness? Ann Intern Med. 1992;117(11):916-21.
Tucker G, Metcalfe A, Pearce C, Need AG, Dick IM, Prince RL, et al. The importance of calculating absolute rather than relative fracture risk. Bone. 2007;41(6):937-41.
Cumpston M, Li T, Page MJ, Chandler J, Welch VA, Higgins JP, et al. Updated guidance for trusted systematic reviews: a new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst Rev. 2019;10:Ed000142
Orsini N, Bellocco R, Greenland S. Generalized Least Squares for Trend Estimation of Summarized Dose–response Data. The Stata Journal. 2006;6(1):40-57.
Greenland S, Longnecker MP. Methods for Trend Estimation from Summarized Dose-Response Data, with Applications to Meta-Analysis. American Journal of Epidemiology. 1992;135(11):1301-9.
Crippa A, Discacciati A, Bottai M, Spiegelman D, Orsini N. One-stage dose-response meta-analysis for aggregated data. Stat Methods Med Res. 2019;28(5):1579-96.
Meikle AW, Tyler FH. Potency and duration of action of glucocorticoids. Effects of hydrocortisone, prednisone and dexamethasone on human pituitary-adrenal function. Am J Med. 1977;63(2):200-7.
Czock D, Keller F, Rasche FM, Häussler U. Pharmacokinetics and pharmacodynamics of systemically administered glucocorticoids. Clin Pharmacokinet. 2005;44(1):61-98.
Webb MSA. Oxford Handbook of Critical Care. 2010
Gauthier J, Wu QV, Gooley TA. Cubic splines to model relationships between continuous variables and outcomes: a guide for clinicians. Bone Marrow Transplant. 2020;55(4):675-80.
Discacciati A, Crippa A, Orsini N. Goodness of fit tools for dose–response meta-analysis of binary outcomes. Research Synthesis Methods. 2017;8(2):149-60.
Sterne JA, Gavaghan D, Egger M. Publication and related bias in meta-analysis: power of statistical tests and prevalence in the literature. J Clin Epidemiol. 2000;53(11):1119-29.
Kang H. Trial sequential analysis: novel approach for meta-analysis. Anesth Pain Med (Seoul). 2021;16(2):138-50.
Crippa A. Multivariate Dose-Response Meta-analysis 2.0.12017. Available from: https://alecri.github.io/software/dosresmeta.html.
Schwarzer G. General Package for Meta-analysis 5.2-02022. Available from: https://github.com/guido-s/meta/.
Schandelmaier S, Briel M, Varadhan R, Schmid CH, Devasenapathy N, Hayward RA, et al. Development of the Instrument to assess the Credibility of Effect Modification Analyses (ICEMAN) in randomized controlled trials and meta-analyses. Cmaj. 2020;192(32):E901-e6.
Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. Bmj. 2008;336(7650):924-6.
Santesso N, Glenton C, Dahm P, Garner P, Akl EA, Alper B, et al. GRADE guidelines 26: informative statements to communicate the findings of systematic reviews of interventions. J Clin Epidemiol. 2020;119:126-35.
Brignardello-Petersen R, Bonner A, Alexander PE, Siemieniuk RA, Furukawa TA, Rochwerg B, et al. Advances in the GRADE approach to rate the certainty in estimates from a network meta-analysis. J Clin Epidemiol. 2018;93:36-44.
Zeng L, Brignardello-Petersen R, Hultcrantz M, Mustafa RA, Murad MH, Iorio A, et al. GRADE Guidance 34: update on rating imprecision using a minimally contextualized approach. Journal of Clinical Epidemiology. 2022;150:216-24.
Lloyd M, Karahalios A, Janus E, Skinner EH, Haines T, De Silva A, et al. Effectiveness of a Bundled Intervention Including Adjunctive Corticosteroids on Outcomes of Hospitalized Patients With Community-Acquired Pneumonia: A Stepped-Wedge Randomized Clinical Trial. JAMA Intern Med. 2019;179(8):1052-60.
Li G, GU C, Zhang S, Lian R, Zhang G. Value of glucocorticoid steroids in the treatment of patients with severe community-acquired pneumonia complicated with septic shock. Chinese Critical Care Med. 2016:780-4.
Nafae RM, Ragab MI, Amany FM, Rashed SB. Adjuvant role of corticosteroids in the treatment of community-acquired pneumonia. Egyptian Journal of Chest Diseases and Tuberculosis. 2013;62(3):439-45.
Sabry NA, Omar EE-D. Corticosteroids and ICU course of community acquired pneumonia in Egyptian settings. Pharmacology & Pharmacy. 2011;2(02):73.
Confalonieri M, Urbino R, Potena A, Piattella M, Parigi P, Puccio G, et al. Hydrocortisone infusion for severe community-acquired pneumonia: a preliminary randomized study. Am J Respir Crit Care Med. 2005;171(3):242-8.
Mikami K, Suzuki M, Kitagawa H, Kawakami M, Hirota N, Yamaguchi H, et al. Efficacy of corticosteroids in the treatment of community-acquired pneumonia requiring hospitalization. Lung. 2007;185(5):249-55.
Meijvis SC, Hardeman H, Remmelts HH, Heijligenberg R, Rijkers GT, van Velzen-Blad H, et al. Dexamethasone and length of hospital stay in patients with community-acquired pneumonia: a randomised, double-blind, placebo-controlled trial. Lancet. 2011;377(9782):2023-30.
Wittermans E, Vestjens SMT, Spoorenberg SMC, Blok WL, Grutters JC, Janssen R, et al. Adjunctive treatment with oral dexamethasone in non-ICU patients hospitalised with community-acquired pneumonia: a randomised clinical trial. Eur Respir J. 2021;58(2)
Snijders D, Daniels JM, de Graaff CS, van der Werf TS, Boersma WG. Efficacy of corticosteroids in community-acquired pneumonia: a randomized double-blinded clinical trial. Am J Respir Crit Care Med. 2010;181(9):975-82.
El-Ghamrawy A, Shokeir M, Esmat A. Effects of low-dose hydrocortisone in ICU patients with severe community-acquired pneumonia. Egyptian Journal of Chest. 2006;55:91-9.
McHardy VU, Schonell ME. Ampicillin dosage and use of prednisolone in treatment of pneumonia: co-operative controlled trial. Br Med J. 1972;4(5840):569-73.
Fernández-Serrano S, Dorca J, Garcia-Vidal C, Fernández-Sabé N, Carratalà J, Fernández-Agüera A, et al. Effect of corticosteroids on the clinical course of community-acquired pneumonia: a randomized controlled trial. Critical Care. 2011;15(2):1-9.
Torres A, Sibila O, Ferrer M, Polverino E, Menendez R, Mensa J, et al. Effect of corticosteroids on treatment failure among hospitalized patients with severe community-acquired pneumonia and high inflammatory response: a randomized clinical trial. Jama. 2015;313(7):677-86.
Blum CA, Nigro N, Briel M, Schuetz P, Ullmer E, Suter-Widmer I, et al. Adjunct prednisone therapy for patients with community-acquired pneumonia: a multicentre, double-blind, randomised, placebo-controlled trial. The Lancet. 2015;385(9977):1511-8.
Marik P, Kraus P, Sribante J, Havlik I, Lipman J, Johnson DW. Hydrocortisone and tumor necrosis factor in severe community-acquired pneumonia: a randomized controlled study. Chest. 1993;104(2):389-92.
Wagner HN, Jr., Bennett IL, Jr., Lasagna L, Cluff LE, Rosenthal MB, Mirick GS. The effect of hydrocortisone upon the course of pneumococcal pneumonia treated with penicillin. Bull Johns Hopkins Hosp. 1956;98(3):197-215.
Meduri GU, Shih MC, Bridges L, Martin TJ, El-Solh A, Seam N, et al. Low-dose methylprednisolone treatment in critically ill patients with severe community-acquired pneumonia. Intensive Care Med. 2022;48(8):1009-23.
Saleem N, Kulkarni A, Chandos Snow TA, Ambler G, Singer M, Arulkumaran N. Effect of corticosteroids on mortality and clinical cure in community-acquired pneumonia: A systematic review, meta-analysis, and meta-regression of randomized control trials. Chest. 2022.
Pitre T, Rochwerg B, Zeraatkar D. Corticosteroids in Community-Acquired Pneumonia: In or Out? CHEST. 2023;163(1):e47-e8.
Annane D, Pastores SM, Rochwerg B, Arlt W, Balk RA, Beishuizen A, et al. Guidelines for the Diagnosis and Management of Critical Illness-Related Corticosteroid Insufficiency (CIRCI) in Critically Ill Patients (Part I): Society of Critical Care Medicine (SCCM) and European Society of Intensive Care Medicine (ESICM) 2017. Critical Care Medicine. 2017;45(12).
Girard TD, Alhazzani W, Kress JP, Ouellette DR, Schmidt GA, Truwit JD, et al. An Official American Thoracic Society/American College of Chest Physicians Clinical Practice Guideline: Liberation from Mechanical Ventilation in Critically Ill Adults. Rehabilitation Protocols, Ventilator Liberation Protocols, and Cuff Leak Tests. Am J Respir Crit Care Med. 2017;195(1):120-33.
Author information
Authors and Affiliations
Contributions
T. P. and D. Z. conceived the study idea. T. P., D. A., and D. Z. screened trials, collected the data, performed the risk of bias assessments, and performed the GRADE assessments. T. P. performed all of the analysis. D. C., S. M. P., A. M. N., D. N., and B. R. provided expert analysis of the data and revised the GRADE assessments. B. R. provided key methodological input and supervision. All authors read and approved the final manuscript. B. R. and D. Z. supervised the study. T. P. is the data guarantor
Ethics declarations
Conflict of Interest:
TP, DC, SMP, AMN, DN, and BR are members of the Society of Critical Care Medicine Corticosteroid Guidelines Focused Update Panel. SMP is the co-Chair of the Society of Critical Care Medicine Corticosteroid Guidelines Focused Update Panel. SMP discloses personal fees for advisory board work from AbbVie, royalty fees from McGraw Hill as textbook editor, and institutional grant support from the National Cancer Institute of the National Institutes of Health under Award Number P30CA008748, RevImmune, BioMerieux, and the Breast Cancer Research Foundation, outside the submitted work. No other authors made any disclosures.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pitre, T., Abdali, D., Chaudhuri, D. et al. Corticosteroids in Community-Acquired Bacterial Pneumonia: a Systematic Review, Pairwise and Dose-Response Meta-Analysis. J GEN INTERN MED 38, 2593–2606 (2023). https://doi.org/10.1007/s11606-023-08203-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11606-023-08203-6