Abstract
Background
Erythrocytosis, most often measured as an increase in hemoglobin and/or hematocrit, is a common reason for referral to internal medicine and hematology clinics and a rational approach is required to effectively identify patients with polycythemia vera while avoiding over-investigation.
Aim
We aimed to develop and validate a simple rule to predict JAK2 mutation positivity based on complete blood count parameters to aid in the diagnostic approach to patients referred for elevated hemoglobin.
Setting
Internal medicine and hematology clinics at an academic tertiary referral center.
Participants
The JAK2 Prediction Cohort (JAKPOT), a large retrospective cohort (n = 901) of patients evaluated by internal medicine and hematology specialists for elevated hemoglobin.
Design
JAK2 mutation analysis was performed in all patients and clinical and laboratory variables were collected. Patients were randomly divided into derivation and validation cohorts. A prediction rule was developed using data from the derivation cohort and tested in the validation cohort.
Key Results
The JAKPOT prediction rule included three variables: (i) red blood cell count >6.45×1012/L, (ii) platelets >350×109/L, and (iii) neutrophils >6.2×109/L; absence of all criteria was effective at ruling out JAK2-positivity with sensitivities 94.7% and 100%, and negative predictive values of 98.8% and 100% in the derivation and validation cohorts, respectively, with an overall low false negative rate of 0.4%. The rule was validated for three different methods of JAK2 testing. Applying this rule to our entire cohort would have resulted in over 50% fewer tests.
Conclusion
In patients with elevated hemoglobin, the use of a simple prediction rule helps to accurately identify patients with a low likelihood of having a JAK2 mutation, potentially limiting costly over-investigation in this common referral population.
Similar content being viewed by others
INTRODUCTION
Erythrocytosis is defined as an increase in concentration of red blood cells (RBCs) above age- and sex-specific reference ranges, most often measured as an increase in hemoglobin and/or hematocrit, both common reasons for referral to internal medicine or hematology specialists for further investigations and management. The differential diagnosis of erythrocytosis is broad and includes an apparent (or relative) increase in RBCs due to decreased plasma volume or a true increased red cell mass (polycythemia). Absolute erythrocytosis or polycythemia can be secondary to underlying conditions such as hypoxia, medications, and erythropoietin secreting tumors or the result of primary bone marrow disorders, namely myeloproliferative neoplasms such as polycythemia vera (PV).
PV has an incidence of 2.7 per 100,000 and is associated with high morbidity and mortality if untreated,1 making it critical to differentiate PV from other causes of erythrocytosis.2 Concerns over underdiagnosis of early-stage PV3 motivated the 2016 revision of the World Health Organization (WHO) classification, which decreased the hemoglobin thresholds to 160 g/L for females and 165 g/L for males.4 These revised thresholds, however, overlap with the normal reference range for healthy adults, raising opposite concerns of potential over-investigation of patients with elevated hemoglobin.5 Indeed, since 2016 our center has seen an increase in referrals for patients with elevated hemoglobin, accompanied by a significant rise in the volume of molecular testing for JAK2 mutations and associated decline in test positivity rate.6, 7 This trend may reflect a combination of factors including adherence to the revised WHO hemoglobin thresholds, improved access to molecular testing, and lack of discriminatory function of conventional tests such as serum erythropoietin (EPO).8
JAK2V617F or exon 12 mutations are present in over 99% of patients with PV.9 However, the frequency of JAK2 mutations in patients with erythrocytosis undergoing molecular testing ranges from 1 to 5% depending on the referral population,10 indicating that the majority of patients investigated for elevated hemoglobin do not have PV. There is currently a lack of consensus on appropriate criteria for molecular testing, which in practice often translates into over-investigation, creating the need for a rational approach to molecular testing.8 Attempts to develop prediction rules to guide JAK2 mutation testing have largely focused on population-based cohorts and have proposed over-inclusive testing criteria, limiting their applicability in clinical practice.13, 14 In this article, we outline a diagnostic approach to patients referred for elevated hemoglobin that uses a simple prediction rule based on complete blood count and white blood cell differential (CBC) parameters to guide JAK2 mutation testing, which we developed and validated in a large retrospective clinical cohort of patients evaluated by internal medicine and hematology specialists (the JAK2 Prediction Cohort).
METHODS
The JAK2 Prediction Cohort (JAKPOT) study was conducted at London Health Sciences Centre, a tertiary referral center which serves a population of approximately 2 million in Southwestern Ontario, Canada. All adult patients referred for elevated hemoglobin (≥160 g/L for females, or ≥165 g/L for males) between January 1, 2015, and May 12, 2021, who underwent JAK2 mutation testing were included. This referral population included patients evaluated by internal medicine and hematology specialists who also performed investigations for possible secondary causes of erythrocytosis. Molecular testing was performed by quantitative polymerase chain reaction (qPCR) using the Roche 480 LightCycler (La Roche AG, Switzerland), single nucleotide polymorphism (SNP) allelotyping using the Agena MassARRAY system (Agena Biosciences, CA, USA), or next-generation sequencing (NGS) using the Oncomine Myeloid Research Assay (ThermoFisher Scientific, MA, USA). qPCR and SNP allelotyping assays tested for JAK2V617F mutations; the NGS assay tested for both JAK2V617F mutations and exon 12 mutations. Age, sex, and CBC parameters at the time of JAK2 mutation testing, including hemoglobin, hematocrit, erythrocytes, leukocytes, neutrophils, platelets, and mean corpuscular volume values were extracted. CBCs were performed on a Sysmex XN Analyzer (Sysmex Corporation, Japan). The JAKPOT study was approved by the Research Ethics Boards at Western University (118139).
Patients were randomly divided into derivation and validation cohorts including all methods of JAK2 mutation testing. JAK2-positive and -negative groups were compared using Student’s t-tests or χ2 tests, as appropriate. Continuous variables were dichotomized at optimal cut-off points using receiver operating characteristic curves. Variables tested included age, sex, hematocrit, and RBC, leukocyte, neutrophil, and platelet counts. Potentially significant predictors were evaluated using multiple variable stepwise logistic regression analysis with JAK2 positivity as the dependent variable with probability for inclusion and removal from the model at 0.05 and 0.10, respectively. The models were evaluated using Hosmer–Lemeshow tests and pseudo-R2 measures. A dichotomous score was derived based on the presence or absence of significant variables and subsequently evaluated and internally validated using logistic regression and χ2 tests using non-parametric bootstrapping with 1000 samples. The final model was tested in the validation cohort and sub-analyses for each method were conducted in a similar fashion. Test accuracy for the criteria was evaluated in both cohorts and all subgroups. Analyses were performed using SPSS Statistics 22 (IBM Corporation, Armonk, NY, USA) and MedCalc® Statistical Software version 20.026 (MedCalc Software Limited, Ostend, Belgium). Further details on analysis are available from the authors upon request.
RESULTS
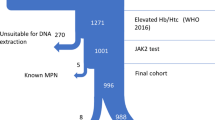
The total study cohort included 901 patients (derivation n = 616, validation n = 285). Population characteristics (Table 1) were similar between derivation and validation cohorts. A positive JAK2 mutation was found in 11.8%, 12.2%, and 10.9% of the total, derivation, and validation cohorts, respectively. Of the variables tested, the final model included the following: RBC count >6.45×1012/L, platelets >350×109/L, and neutrophils >6.2×109/L. Patients with none of these criteria were considered low-risk; patients with any of these criteria were considered high-risk. In the derivation cohort, the percentages of JAK2-positive results in low- and high-risk patients were 1.2% and 24.9%, respectively. The model had a sensitivity of 94.7% and a negative predictive value of 98.8%. Results were consistent in the validation cohort, where the model had a sensitivity of 100% and negative predictive value of 100%. Table 2 shows contingency tables for each cohort. Overall, the false negative rate in our study was 0.4%. Results were also consistent across each testing method (Supplemental Table 1). Based on this analysis, we developed a testing algorithm to guide JAK2 mutation testing using our model (Fig. 1). Applying this rule to our entire cohort of patients would have resulted in over 50% fewer tests.
DISCUSSION
PV is a myeloproliferative neoplasm associated with high morbidity and mortality if untreated. PV has a range of presentations, with classic clinical features including aquagenic pruritus, splenomegaly, and unexplained thrombosis; however, initial referral to internal medicine or hematology is most commonly triggered by an elevated RBC count, elevated hemoglobin and/or elevated hematocrit. Comprehensive evaluation of patients with erythrocytosis can include extensive investigations for secondary causes (Fig. 2) in addition to more costly, specialized testing such as JAK2 mutation analysis. The high sensitivity and specificity of JAK2 mutation analysis makes it the preferred test in patients with suspected PV resulting in this test being performed up front in many settings.9 However, there remains a need for tools to more effectively utilize JAK2 testing in patients referred for erythrocytosis in order to reduce indiscriminate use of costly molecular testing.8
Proposed rational diagnostic approach to the investigation of patients referred for erythrocytosis. Note: this is a proposed approach to the investigation of erythrocytosis demonstrating how the JAKPOT rule might be used to identify low-risk patients; this approach is provisional and requires prospective validation before implementation in routine clinical practice. †Some advocate red cell mass studies to establish absolute vs. relative erythrocytosis, although this test is not widely available and not routinely performed. ‡JAK2 mutation testing can be performed polymerase chain reaction (qPCR), single nucleotide polymorphism (SNP) allelotyping, or next-generation sequencing (NGS). §Serum EPO measurement can be considered an ancillary test to evaluate for primary vs. secondary causes of erythrocytosis, although results are often unavailable at time of initial consultation and can be normal in patients with PV; EPO likely has limited added diagnostic value in settings where JAK2 mutation testing is available. ¶Note: bone marrow biopsy demonstrating “panmyelosis” is a major criterion and “subnormal” serum EPO level a minor criterion in the World Health Organization 2016 diagnostic criteria for polycythemia vera. Abbreviations: EPO, erythropoietin; SGLT2, sodium-glucose cotransporter-2; JAK2, Janus Kinase 2; JAKPOT, JAK2 Prediction Cohort.
The initial diagnostic approach should include evaluation for absolute versus relative erythrocytosis and clinical assessment for common secondary causes such as smoking, hypoxic lung disease, and medications (Fig. 2). If identified, management should be directed at the secondary cause with monitoring for resolution of erythrocytosis. In patients where no clear secondary cause is identified, the next step is to determine the likelihood of PV and whether JAK2 molecular testing may be necessary. Traditionally, serum EPO has been used to assist in decision-making, with low EPO being associated with PV versus high EPO in secondary erythrocytosis; however, recent evidence suggests serum EPO measurement is often unreliable and can be normal in over a third of patient with PV.8 Moreover, EPO levels are rarely available to guide decision-making at the time of initial consultation and measurement can further contribute to diagnostic delays.
To assist in the decision to order JAK2 mutation testing, we propose a simple prediction rule, the JAKPOT rule, which uses CBC parameters to predict the likelihood of JAK2 mutation positivity in patients presenting with elevated hemoglobin. We developed and validated this prediction rule in a large cohort of patients referred to internal medicine and hematology specialists for erythrocytosis where it demonstrated a high negative predictive value (>98.8%). The proposed rule demonstrates improved sensitivity and negative predictive value compared to low serum EPO in ruling out a diagnosis of PV in a similar patient population.8 This rule could help limit indiscriminate over-investigation of patients referred for erythrocytosis and help optimize the diagnostic yield of molecular testing. In our cohort, implementation of this rule could have significantly reduced the number of tests for JAK2 mutations with minimal risk of missing diagnoses of JAK2-positive PV.
The three parameters included in the JAKPOT rule are RBC count, platelet count, and neutrophil count, which have well-established pathophysiologic rationale given that elevations in all of these values (i.e., panmyelosis) are a recognized biological hallmark of PV.11 If either the RBC count, platelet count, or neutrophil count meet their respective thresholds as outlined in the JAKPOT rule, then molecular diagnostic testing for JAK2 mutations is recommended. Whereas hemoglobin and hematocrit are independently affected by cell size, plasma volume, and iron stores, RBC count provides a more accurate indicator of erythropoiesis and red cell mass.12 Despite this, RBC count has not been incorporated in other prediction rules or diagnostic criteria for PV. It is also known that patients with so-called masked PV—“masked” due to concurrent iron deficiency—can initially present with thrombocytosis.3 Platelet count has been used in other algorithms;13 however, our model uses a higher threshold of 350×109/L than other algorithms, increasing the discriminatory power.
Our approach has several strengths. First, it uses readily available CBC parameters making it easy to apply at initial clinic visits without introducing delays required for other assays such as serum EPO. Importantly, these parameters are objective and not subject to interpretation with its inherent bias. Additionally, this prediction rule was derived from a real-world cohort of patients referred for elevated hemoglobin as opposed to a population-based cohort,14 and validated across three different assays for JAK2 mutation testing, including qPCR, SNP allelotyping, and NGS platforms. Most importantly, our approach uses a Bayesian decision framework that increases the diagnostic accuracy and safety by applying this rule only to patients with a higher pre-test probability of having PV as defined by the hemoglobin threshold accepted by the WHO 2016 diagnostic criteria. The use of the rule allows to identify those patients with a very low posterior probability of having a positive JAK2 mutation with a very high level of accuracy. This strategy has proven to be highly successful in other areas of medicine. The best example is the use of the Wells’ clinical prediction rules for deep vein thrombosis and pulmonary embolism that, in conjunction with the results of a D-dimer, allow clinicians to safely rule out these potentially serious diagnoses with a very high level of accuracy.15, 16
Our prediction rule offers several advantages over others in the literature.13, 14 Piris-Villaespesa et al.13 recently proposed an algorithm for PV screening using platelet and neutrophil counts but lacked JAK2 status for all patients in the validation cohort. Mahe et al.14 developed a decision tree using CBC parameters to guide JAK2 mutation testing based on data from the Copenhagen General Population Study. While this algorithm had a high negative predictive value of 92% and a false-negative rate of only 1.2%, it recommended JAK2 mutation testing in any patient with a hemoglobin >160 g/L (>165 g/L in men), a platelet count >350×109/L, or white blood cell count >7×109/L and thus has limited ability to reduce molecular testing in patients with abnormal CBC parameters. Given that this rule recommends testing in all patients with elevated hemoglobin level (>160 g/L in women and >165 g/L in men), nearly all patients in our cohort would have been eligible for testing, limiting its applicability in clinical practice.
There are several limitations to our study. Our prediction rule was derived and validated retrospectively using data from a single referral center, which may limit its generalizability to other centers and populations. Further studies to prospectively validate this prediction rule in different populations at other centers are planned. While our cohort included all patients with elevated hemoglobin who underwent JAK2 mutation analysis, it did not capture patients who did not have JAK2 testing performed. Nonetheless, during the study period it was standard practice at our center to perform JAK2 mutation testing in patients referred for elevated hemoglobin, and, moreover, this cohort better reflects the more clinically relevant, potentially higher risk population to whom our prediction rule is most applicable. The different methods for JAK2 mutation testing used in our study had varying sensitivities, with qPCR, SNP allelotyping, and NGS detecting allele frequencies as low as 0.01%, 5%, and 5% respectively and detection of JAK2 exon 12 mutations was limited to the NGS assay. Despite these differences, the model showed consistent performance across all methods of JAK2 testing suggesting it may be generalizable to centers using different testing platforms, although this remains to be demonstrated. Additional clinical variables, such as medications, history of arterial or venous thrombosis, and splenomegaly, were not considered in our model but could potentially further improve to predictive value of this rule. Given this study’s retrospective nature, we were unable to evaluate follow-up CBCs and their impact on the likelihood of JAK2 positivity; however, such variables might also be used in future prediction rules to further improve sensitivity.
In conclusion, the differential diagnosis for patients presenting with erythrocytosis is broad; PV, albeit rare, must be considered due to its high morbidity and mortality. We outlined a rational diagnostic approach to erythrocytosis (Fig. 2), which includes a simple prediction rule to guide JAK2 mutation testing using readily available CBC parameters (Fig. 1). As with all clinical prediction rules, this rule is not meant to replace clinical judgment but could be used as a guide to help limit over-investigation in a common referral population. Such a rule could help improve resource stewardship by avoiding unnecessary and costly testing, in line with other Choosing Wisely initiatives.13
References
Marchioli, R. et al. Vascular and neoplastic risk in a large cohort of patients with polycythemia vera. J Clin Oncol. 205;23:2224-2232.
Mithoowani, S., Laureano, M., Crowther, M. A. & Hillis, C. M. Investigation and management of erythrocytosis. CMAJ. 2020;192:E913-E918.
Barbui T, Thiele J, Gisslinger H, et al. Masked polycythemia vera (mPV): results of an international study. Am J Hematol. 2014;89:52-54.
Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127:2391-2405.
Sandes AF, Gonçalves MV, Chauffaille ML. Frequency of polycythemia in individuals with normal complete blood cell counts according to the new 2016 WHO classification of myeloid neoplasms. Int J Lab Hematol. 2017;39:528-531.
Chin-Yee B, Matyashin M, Bhai P, et al. Investigating erythrocytosis: changing practice patterns in the era of molecular diagnostics. Blood. 2021;138(Supplement 1):4630-4630.
Chin-Yee B, Matyashin M, Cheong I, Bhai P, Lazo-Langner A, Almanaseer A, Kawata E, Levy MA, Stuart A, Lin H, Chin-Yee I, Sadikovic B, Hsia C. Secondary causes of elevated hemoglobin in patients undergoing molecular testing for suspected polycythemia vera in southwestern Ontario: a chart review. CMAJ Open. 2022;10(4)IE988-E992. https://doi.org/10.9778/cmajo.20210322.
Chin-Yee, B, Cheong C, Bhai P, et al. Serum erythropoietin levels in 696 patients investigated for erythrocytosis with JAK2 mutation analysis. Am J Hematol. 2022;Jan 19.
Tefferi, A. & Barbui, T. Polycythemia vera and essential thrombocythemia: 2021 update on diagnosis, risk-stratification and management. Am J Hematol 2020;95:1599-1613.
Wouters HJCM, Mulder R, van Zeventer IA, et al. Erythrocytosis in the general population: clinical characteristics and association with clonal hematopoiesis. Blood Adv. 2020;4:6353-6363.
Spivak JL. How I treat polycythemia vera. Blood. 2019;134:341-352.
Hasselbalch HC. Time for revival of the red blood cell count and red cell mass in the differential diagnosis between essential thrombocythemia and polycythemia vera. Haematologica. 2019;104:2119-2125.
Piris-Villaespesa M, Álvarez-LarrÁn A, Saez-Marín A, et al. Development and validation of a sequential two-step algorithm for the screening of individuals with potential polycythaemia vera. Sci Rep. 2021;11:209.
Mahe E, Pedersen KM, Çolak Y, et al. JAK2-tree: a simple CBC-based decision rule to guide appropriate JAK2 V617F mutation testing. J Clin Pathol. 2019;72:172-176.
Wells PS, Anderson DR, Roger M, et al. Excluding pulmonary embolism at the bedside without diagnostic imaging: management of patients with suspected pulmonary embolism presenting to the emergency department by using a simple clinical model and d-dimer. Ann Intern Med. 2001;135:98-107.
Wells PS, Anderson DR, Roger M, et al. Evaluation of D-dimer in the diagnosis of suspected deep-vein thrombosis. N Engl J Med. 2003;349:1227-1235.
Author information
Authors and Affiliations
Contributions
This study was designed by B.C.Y., A.L.L., I.C.Y., and C.C.H. Molecular diagnostic data were provided by B.S., P.B., M.A.L., A.S., and H.L. Clinical and laboratory data were collected by I.C., M.M, E.K. B.C.Y., C.C.H, and I.C.Y. Data analysis and interpretation was performed by A.L.L., I.C., B.C.Y., M.K., and J.M.H. B.C.Y. wrote the paper with input from all authors who approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they do not have a conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOCX 33 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chin-Yee, B., Bhai, P., Cheong, I. et al. A Rational Approach to JAK2 Mutation Testing in Patients with Elevated Hemoglobin: Results from the JAK2 Prediction Cohort (JAKPOT) Study. J GEN INTERN MED 38, 1828–1833 (2023). https://doi.org/10.1007/s11606-022-07963-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11606-022-07963-x