Abstract
Background
Previous meta-analyses of the benefits and harms of glucagon-like peptide-1 receptor agonists (GLP1RAs) have been limited to specific outcomes and comparisons and often included short-term results. We aimed to estimate the longer-term effects of GLP1RAs on cardiovascular risk factors, microvascular and macrovascular complications, mortality, and adverse events in patients with type 2 diabetes, compared to placebo and other anti-hyperglycemic medications.
Methods
We searched PubMed, Scopus, and clinicaltrials.gov (inception–July 2019) for randomized controlled trials ≥ 52 weeks’ duration that compared a GLP1RA to placebo or other anti-hyperglycemic medication and included at least one outcome of interest. Outcomes included cardiovascular risk factors, microvascular and macrovascular complications, all-cause mortality, and treatment-related adverse events. We performed random effects meta-analyses to give summary estimates using weighted mean differences (MD) and pooled relative risks (RR). Risk of bias was assessed using the Cochrane Collaboration risk of bias in randomized trials tool. Quality of evidence was summarized using the Grading of Recommendations, Assessment, Development, and Evaluation approach. The study was registered a priori with PROSPERO (CRD42018090506).
Results
Forty-five trials with a mean duration of 1.7 years comprising 71,517 patients were included. Compared to placebo, GLP1RAs reduced cardiovascular risk factors, microvascular complications (including renal events, RR 0.85, 0.80–0.90), macrovascular complications (including stroke, RR 0.86, 0.78–0.95), and mortality (RR 0.89, 0.84–0.94). Compared to other anti-hyperglycemic medications, GLP1RAs only reduced cardiovascular risk factors. Increased gastrointestinal events causing treatment discontinuation were observed in both comparisons.
Discussion
GLP1RAs reduced cardiovascular risk factors and increased gastrointestinal events compared to placebo and other anti-hyperglycemic medications. GLP1RAs also reduced MACE, stroke, renal events, and mortality in comparisons with placebo; however, analyses were inconclusive for comparisons with other anti-hyperglycemic medications. Given the high costs of GLP1RAs, the lack of long-term evidence comparing GLP1RAs to other anti-hyperglycemic medications has significant policy and clinical practice implications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
BACKGROUND
Type 2 diabetes (T2D) affects more than 30 million adults in the USA and over 9% of the worldwide population.1 Patients with T2D are at an increased risk of atherosclerotic cardiovascular disease (ASCVD) and develop ASCVD approximately 15 years earlier compared to patients without T2D.2 Recently, results from several large randomized trials of the glucagon-like peptide-1 receptor agonists (GLP1RAs) have shown that, in addition to lowering hemoglobin A1c (HbA1c), GLP1RAs may also reduce ASCVD.
However, not all of these large trials comparing GLP1RAs to placebo demonstrated cardiovascular benefit. Reductions in ASCVD, as measured by primary major adverse cardiovascular event (MACE) outcomes, were observed in LEADER3 (liraglutide), SUSTAIN-64 (semaglutide), and REWIND5 (dulaglutide) for GLP1RAs, compared to placebo. Additionally, LEADER demonstrated reduced rates of cardiovascular death and all-cause mortality with liraglutide compared to placebo.3 In contrast, no differences in MACE outcomes were found in ELIXA6 (lixisenatide) or EXSCEL7 (exenatide).
Many systematic reviews have attempted to quantify the efficacy of GLP1RAs given these discrepant results. However, the scope of these previous reviews has been limited. The majority of reviews have examined only one or two categories of clinically important outcomes (either cardiovascular risk factors,8,9,10,11,12,13,14 microvascular outcomes,15 MACE,16,17,18,19 mortality,17 or adverse events9,11,12,20). Furthermore, the majority of these reviews restricted inclusion to comparisons with placebo12,18,19,20,21 or specific diabetic drugs (metformin,22 insulin,11,14 within GLP1RA class comparisons,12,23 dipeptyl peptidase-4 inhibitor (DPP4I),19 sodium-glucose co-transporter-2 inhibitor19), thereby eliminating many clinical trial results from consideration. Finally, prior reviews included trials with short follow-up times (≤ 6 months),8,9,10,11,12,13,14,16,17,18,19,20,23,24,25,26 which report benefits that may or may not be sustained and may underestimate adverse events that accrue over time.27,28
To date, a comprehensive review of longer-term benefits and harms of GLP1RAs vs. placebo and other anti-hyperglycemic medications has not been published. Therefore, we conducted a meta-analysis of all randomized trials of GLP1RAs compared to placebo or other anti-hyperglycemic medications for patients with T2D, with at least 52-week study duration, and that reported cardiovascular risk factor changes, microvascular or macrovascular complications, all-cause mortality, or treatment-related adverse events.
METHODS
The study protocol is reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA)29 and was registered a priori with the International Prospective Register of Systematic Reviews (PROSPERO, CRD42018090506)30 database (eTable 1).
A systematic search of PubMed, Scopus, and clinicaltrials.gov was conducted from inception to July 2019. No language restrictions were used. Details of the search terms are available in eTable 2. We used search terms reflecting the words “diabetes” and either “glucagon-like peptide 1” or individual GLP1RA drug names. After removing duplicates, articles that were not relevant were excluded by title, abstract, or full-text review by two reviewers. If there were disagreements during title or abstract review, articles were automatically moved to full-text review. During full-text review, disagreements were resolved by consensus. Finally, a hand search of citations from published systematic reviews was completed.
Studies were eligible if they were randomized controlled trials with (1) treatment comparisons of GLP1RA vs. placebo and/or other anti-hyperglycemic medications, (2) duration of at least 52 weeks, (3) adults age 18 years or older with T2D, and (4) at least one outcome of interest.
Outcomes of interest included cardiovascular risk factors, microvascular and macrovascular complications, all-cause mortality, and treatment-related adverse events. Cardiovascular risk factors included HbA1C, systolic blood pressure (SBP), heart rate, body mass index (BMI), weight, low-density lipoprotein (LDL), high-density lipoprotein (HDL), and estimated glomerular filtration rate (eGFR). Macrovascular complications included myocardial infarction (MI), heart failure, stroke, and composite macrovascular complications (three-, five-, and six-component MACE outcomes) (eTable 3). Microvascular complications included retinopathy, blindness, foot ulcer, end-stage renal disease (ESRD), or any renal event (eTable 3). We included all adverse events, but report results for adverse events with sufficient number of trials to make inferences: any hypoglycemia, severe hypoglycemia, gastrointestinal (GI) events leading to treatment discontinuation, any pancreatitis, pancreatic cancer, medullary thyroid cancer, and bone fracture (eTable 3). Due to inconsistencies in how cardiovascular death was defined across trials, we did not analyze cardiovascular death as an outcome.
Data were independently extracted and quality of evidence was judged by two of five reviewers (J.A., E.S., M.F., A.K., and N.L.). Data were extracted for all outcomes at all study follow-up time periods. Discrepancies were resolved by a third reviewer and discussion if necessary. To assess risk of bias, we utilized the Cochrane Collaboration risk of bias in randomized trials tool with five bias domains (sequence generation, blinding, attrition, detection, and reporting).31 For attrition bias, we assigned less than 10% loss to follow-up as low risk of bias, and judged higher rates as either moderate or high risk based on likelihood of threatening the internal validity of the results.32 Studies were judged to have an overall high risk of bias if one or more domains were high risk, or if three or more domains were moderate risk for bias. Quality of evidence across trials was synthesized using the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) approach for the seven most clinically relevant outcomes (HbA1c, mortality, 3-component MACE, stroke, MI, renal events, and adverse GI events).
We applied the following rules for our synthesis. For trials reporting results for more than one time period, we included results for the period with the lowest risk of bias for HbA1c. If a trial had the same risk of bias for more than one follow-up period, then the results from the longest follow-up period was included. If a trial had study arms with different drug dosages, we included the study arm that matched the dosage level in the comparison group and most closely matched the other included trials. If the comparison group was placebo or had an unspecified dosage, we included the higher dose study arm. Finally, if drugs were titrated per protocol, we used the maximum allowable dose to categorize the dosage level (eTable 4).
Statistical Analysis
When there were at least two trials, we performed pooled analyses using random effects models. Weighted mean differences (MDs) were calculated for continuous outcomes, and pooled relative risks (RRs) were calculated for dichotomous outcomes. For RR calculations where no events were reported, we added a 0.5 correction.33 When evaluating binary outcomes, we preferentially used event rates where possible, and if unavailable, we used hazard ratios. Trial heterogeneity was assessed subjectively and with the I2 statistic. Publication bias was assessed by funnel plots, and for outcomes with at least 10 studies, with Egger’s and Begg’s tests.34
For outcomes in which there were at least 10 studies35 and the I2 was ≥ 50% we explored heterogeneity by using subgroup analyses. Subgroup analyses varied by outcome and were determined based on authors’ clinical judgment (J.A. and N.L.). Subgroup analyses included restricting analyses to (1) trials with high dosages of GLP1RAs, (2) trials in which GLP1RAs were combined with background anti-hyperglycemic medication, (3) trials in which GLP1RAs did not include a standard care approach where other anti-hyperglycemic medications were permitted in addition to the study drug, and stratifying trials by (4) the percentage of their population with ASCVD, (5) study duration, (6) baseline HbA1c, and (7) baseline BMI. For comparisons of GLP1RAs vs. other anti-hyperglycemic medications, subgroup analyses by other anti-hyperglycemic medications class were also conducted. SAS version 9.4 software was used for all analyses.
RESULTS
Search Results
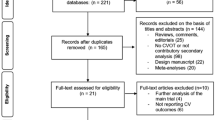
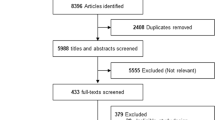
Of the 39,396 articles identified, 55 articles comprising 45 trials (n=71,517) met inclusion criteria (Figure 1). Among the included trials, 17 trials (n=61,330) compared GLP1RAs vs. placebo (eTable 5) and 30 trials (n=19,785) compared GLP1RAs vs. other anti-hyperglycemic medications (eTable 6). Two trials had comparisons with both placebo and another anti-hyperglycemic medication.
Among placebo-controlled trials, six of the 17 placebo-controlled trials required participants to have a high risk for or pre-existing ASCVD (eTable 7). Patients tended to be in their fifth or sixth decade of life, white, male, and obese (mean BMI ranged from 30 to 35 in 14 of 16 trials that reported mean BMI), with a baseline HbA1c ranging from 7 to 9% and median diabetes duration of more than 6 years (eTable 8).
In trials comparing GLP1RAs vs. other anti-hyperglycemic medications, patient characteristics were similar to placebo-controlled trials; however, Asian race was more common because five trials were conducted exclusively in Asia (eTable 9). The comparison group was insulin in 12 trials, a DPP4I in nine trials, a sulfonylurea in five trials, and other drugs in four trials. Only two of these trials required pre-existing ASCVD (eTable 7). These trials more often included cardiovascular risk factors, mortality, and adverse event outcomes compared to placebo-controlled trials (eTables 10 and 11).
The quality of evidence across trials using the GRADE approach for the seven most clinically relevant outcomes is summarized in Table 1. Details about the risk of bias are available in several eTables (eTables 12–24). In general, the risk of bias due to sequence generation, detection, and reporting was judged as low for the majority of the included studies. For studies in which the overall risk of bias was judged as moderate or high risk, the most common reason was due to attrition. Among placebo-controlled trials, the overall quality of evidence was judged as high for six of the seven outcomes, with HbA1c receiving a moderate overall rating due to the presence of attrition bias. In trials comparing GLP1RAs to other anti-hyperglycemic medications, the overall quality of evidence ratings was more variable. In particular, risk of bias and imprecision led to low quality of evidence ratings for the outcomes of myocardial infarction, stroke, and any renal event. No publication bias was detected for any of the seven outcomes for either comparison (eFigures 1–4).
Cardiovascular Risk Factors
GLP1RAs led to lower HbA1c levels compared to both placebo (MD = −0.67%, 95% Cl −0.77 to −0.58%, I2 = 93%) and other anti-hyperglycemic medications (−0.37%, −0.53 to −0.22%, I2 = 93%) (Figures 2 and 3, eTable 25). Similar reductions in HbA1c favoring GLP1RAs were observed in trials where participants received background medications at baseline, and in trials where participants were not permitted to receive other anti-hyperglycemic medications in addition to the study drug (eTable 26). GLP1RAs also reduced SBP (vs. placebo, −1.75 mmHg, −2.14 to −1.35 mmHg, I2 = 48%; vs. other anti-hyperglycemic medications, −1.90 mmHg, −2.57 to −1.22 mmHg, I2 = 32%), weight (vs placebo, −1.84 kg, −2.37 to −1.30 kg, I2 = 95%; vs other anti-hyperglycemic medications, −3.39 kg, −4.13 to −2.66 kg, I2 = 94%), BMI (vs placebo, −1.12 kg/m2, −1.67 to −0.57 kg/m2, I2 = 96%; vs other anti-hyperglycemic medications, −2.07 kg/m2, −2.74 to −1.39 kg/m2, I2 = 96%), and LDL (vs placebo, −0.04 mmol/L, −0.06 to −0.02, I2 = 0%; vs other anti-hyperglycemic medications, −0.04 mmol/L, −0.09 to 0 mmol/L, I2 = 0%). Heart rate increased by about 2 bpm (vs. placebo, 2.22 bpm, 1.69 to 2.75 bpm, I2 = 88%; vs. other anti-hyperglycemic medication, 1.81 bpm, 1.23 to 2.39 bpm, I2 = 57%). In subgroup analyses of cardiovascular risk factors, results did not vary (eTable 26 and eTable 27), except GLP1RAs led to larger reductions in HbA1c when compared to DPP4Is, had greater reductions in SBP when compared to sulfonylureas or insulin, and greater reductions in weight when compared to sulfonylureas, TZDs, or insulin.
Macrovascular and Microvascular Outcomes
The 3-component MACE outcome favored GLP1RAs compared to placebo (RR = 0.87, 0.82 to 0.93, I2 = 23%). GLP1RAs led to fewer strokes (0.86, 0.78 to 0.95, I2 = 0%) and renal events (0.85, 0.80 to 0.90, I2 = 0%) compared to placebo (Figure 4; eTable 25). No differences in 5- or 6-component MACE, fatal or non-fatal MI, heart failure, retinopathy, or blindness were observed. No differences in any macrovascular or microvascular outcomes were observed between GLP1RAs and other anti-hyperglycemic medications, although the number of patients analyzed for each outcome was limited (Figure 5; eTable 25). Results were consistent across subgroup analyses (eTable 26 and eTable 27).
Mortality
When compared to placebo, GLP1RAs had a lower risk of death (RR = 0.89, 0.84 to 0.94, I2 = 0%). Mortality was not different between GLP1RAs and other anti-hyperglycemic medications (RR = 0.68, 0.42 to 1.11, I2 = 0%). Subgroup analyses yielded consistent results (eTables 26 and 27).
Adverse Events
Compared to both placebo and other anti-hyperglycemic medications, GLP1RAs had more frequent GI events (RR = 3.84, 2.59 to 5.70, I2 = 52%; 3.32, 1.95 to 5.65, I2 = 42%, respectively) (Figures 4 and 5; eTable 25). Comparing GLP1RAs to placebo, no differences in any or severe hypoglycemic events were observed, but in comparisons to other anti-hyperglycemic medications, GLP1RAs were less likely associated with any or severe hypoglycemic events (RR = 0.73, 0.62 to 0.86; 0.59, 0.37 to 0.95). In subgroup analyses by drug class, GLP1RAs led to fewer hypoglycemic events in comparisons with sulfonylureas (RR = 0.33, 0.22 to 0.49) and insulin (RR = 0.67, 0.56 to 0.80), but there was no difference with DPP4Is (RR = 1.23, 0.85 to 1.78). No differences with pancreatic cancer, medullary thyroid cancer, or pancreatitis were observed.
DISCUSSION
Our systematic review and meta-analysis is the first to comprehensively assess the long-term safety and efficacy of GLP1RAs compared to placebo, and the first to compare the effectiveness to other anti-hyperglycemic drugs overall. We found that GLP1RAs compared to placebo were associated with at least 1-year reductions in cardiovascular risk factors (including HbA1c, weight, BMI, SBP, and HR), renal events, macrovascular outcomes (stroke and 3-component MACE), and mortality, and increases in GI adverse events. In comparisons to other anti-hyperglycemic medications, GLP1RAs were associated with reductions in cardiovascular risk factors, increases in GI adverse events, and fewer hypoglycemic events than sulfonylureas and insulin. No difference in mortality was observed. Furthermore, we did not find sufficient data allowing conclusions on how GLP1RAs compare to other anti-hyperglycemic medications for microvascular outcomes or macrovascular outcomes.
In clinical practice, the major clinical decision is often not whether to initiate a diabetes medication but rather which diabetes medication to select. As such, the clinical relevance of the lack of reductions in microvascular outcomes, macrovascular outcomes, or mortality in comparisons of GLP1RAs to other anti-hyperglycemic medications is important. Of the 30 trials included for this comparison, the vast majority were designed to assess cardiovascular risk factors, particularly HbA1c, over time and not more clinically meaningful outcomes. Therefore, small sample size, high attrition, and serious imprecision limited our assessment of microvascular and macrovascular outcomes for comparisons of GLP1RAs to other anti-hyperglycemic medications. While it stands to reason that long-term reductions in cardiovascular risk factors may lead to reductions in microvascular complications, macrovascular complications, or mortality, our results show that evidence for these benefits from randomized controlled trials over 1 year does not yet exist. Our results may temper excitement surrounding the possible cardiovascular benefits of GLP1RAs when comparing them to other anti-hyperglycemic medications.36 Longer observational periods after these trials may be necessary to observe any reductions in cardiovascular complications or mortality. Currently, these results suggest that side effect profile, cost, and patient preference should continue to play a crucial role in shared decision-making conversations with patients when considering second-line agents for T2D.37
Compared to placebo, GLP1RAs were associated with small absolute cardiovascular risk factor changes and favorable reductions in microvascular outcomes, macrovascular outcomes, and mortality. The effect estimates we found confirm findings from prior meta-analyses and provide reassurance that GLP1RA benefits vs. placebo are sustained at 1 year.
In comparisons with other anti-hyperglycemic medications, GLP1RAs reduced multiple cardiovascular risk factors. Importantly, the magnitude of effect was often related to known effects of the other anti-hyperglycemic medications to which GLP1RAs were being compared. For example, sulfonylureas and insulin are known to cause weight gain, and therefore, reductions in weight were more pronounced when GLP1RAs were compared to sulfonylureas (−5.37 kg), TZDs (−4.85 kg), and insulin (−4.13 kg), than with DPP4Is (−2.13 kg). Regarding HbA1c, use of GLP1RAs led to larger reductions when compared to DPP-4Is (−0.62%) rather than sulfonylureas (−0.28%) or insulin (−0.22%). These comparisons inform shared decision-making discussions when considering GLP1RAs alongside other anti-hyperglycemic medications for patients with T2D.
Consistent with clinical practice, GLP1RAs increased GI adverse events compared to placebo and other anti-hyperglycemic medications. GLP1RAs were associated with lower rates of hypoglycemia when compared to other anti-hyperglycemic medications, mostly due to comparisons with insulin and sulfonylureas. While we did not find that GLP1RAs were associated with pancreatitis, pancreatic cancer, or medullary thyroid cancer, our results were not adequately powered to exclude an association, if one exists.
This systematic review and meta-analysis has several limitations. First, we chose to include trials that lasted at least 52 weeks. This may have minimized effect sizes seen with short-term outcomes such as HbA1c and weight and may also bias longer-term outcomes such as mortality towards the null by including trials not designed to assess these outcomes. However, we believe 52 weeks is an important benchmark clinicians use when weighing the benefits and risks of prescribing GLP1RAs. Second, we limited our quality of evidence evaluation to seven of the 25 outcomes we reported. While this decision limits the interpretation of some results, we focused our quality assessment on the most pertinent outcomes that factor into the clinical decision-making of prescribing GLP1RAs to patients with T2D. Third, significant heterogeneity was present among several of the outcomes we analyzed, particularly among the cardiovascular risk factors. Results from subgroup analyses, however, yielded consistent results, and the directionality of the effects of these comparisons were consistent across trials. Fourth, we only included randomized trials in our review which tended to include predominantly white populations and included few patients over the age of 75, which may limit the generalizability of results to real-world practice.
CONCLUSIONS
Our systematic review and meta-analysis is the first to synthesize findings related to long-term use of GLP1RAs compared to placebo and other anti-hyperglycemic medications. GLP1RAs compared to placebo were associated with significant reductions in cardiovascular risk factors, renal events, stroke, 3-component MACE, and mortality. GLP1RAs compared to other anti-hyperglycemic medications were associated with reductions in cardiovascular risk factors. Insufficient evidence exists to evaluate the long-term effects of GLP1RAs compared to other anti-hyperglycemic medications on microvascular or macrovascular outcomes. These findings inform decisions on benefits and tradeoffs when prescribing GLP1RAs for individual patients with T2D.
References
Saeedi P, Petersohn I, Salpea P, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9(th) edition. Diabetes Research and Clinical Practice. 2019;157:107843.
Low Wang CC, Hess CN, Hiatt WR, Goldfine AB. Clinical Update: Cardiovascular Disease in Diabetes Mellitus: Atherosclerotic Cardiovascular Disease and Heart Failure in Type 2 Diabetes Mellitus - Mechanisms, Management, and Clinical Considerations. Circulation. 2016;133(24):2459-2502.
Marso SP, Daniels GH, Brown-Frandsen K, et al. Liraglutide and Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med. 2016;375(4):311-322.
Marso SP, Bain SC, Consoli A, et al. Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes. N Engl J Med. 2016;375(19):1834-1844.
Gerstein HC, Colhoun HM, Dagenais GR, et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet (London, England). 2019.
Pfeffer MA, Claggett B, Diaz R, et al. Lixisenatide in Patients with Type 2 Diabetes and Acute Coronary Syndrome. New Engl J Med. 2015;373(23):2247-2257.
Holman RR, Bethel MA, Mentz RJ, et al. Effects of Once-Weekly Exenatide on Cardiovascular Outcomes in Type 2 Diabetes. New Engl J Med. 2017;377(13):1228-1239.
Vilsbøll T, Christensen M, Junker AE, Knop FK, Gluud LL. Effects of glucagon-like peptide-1 receptor agonists on weight loss: systematic review and meta-analyses of randomised controlled trials. BMJ (Clinical research ed). 2012;344:d7771.
Liakopoulou P, Liakos A, Vasilakou D, et al. Fixed ratio combinations of glucagon like peptide 1 receptor agonists with basal insulin: a systematic review and meta-analysis. Endocrine. 2017;56(3):485-494.
Eng C, Kramer CK, Zinman B, Retnakaran R. Glucagon-like peptide-1 receptor agonist and basal insulin combination treatment for the management of type 2 diabetes: a systematic review and meta-analysis. Lancet (London, England). 2014;384(9961):2228-2234.
Singh S, Wright EE, Jr., Kwan AY, et al. Glucagon-like peptide-1 receptor agonists compared with basal insulins for the treatment of type 2 diabetes mellitus: a systematic review and meta-analysis. Diabetes, obesity & metabolism. 2017;19(2):228-238.
Htike ZZ, Zaccardi F, Papamargaritis D, Webb DR, Khunti K, Davies MJ. Efficacy and safety of glucagon-like peptide-1 receptor agonists in type 2 diabetes: A systematic review and mixed-treatment comparison analysis. Diabetes, obesity & metabolism. 2017;19(4):524-536.
Waldrop G, Zhong J, Peters M, et al. Incretin-based therapy in type 2 diabetes: An evidence based systematic review and meta-analysis. Journal of diabetes and its complications. 2018;32(1):113-122.
Castellana M, Cignarelli A, Brescia F, Laviola L, Giorgino F. GLP-1 receptor agonist added to insulin versus basal-plus or basal-bolus insulin therapy in type 2 diabetes: A systematic review and meta-analysis. Diabetes Metab Res Rev. 2019;35(1):e3082.
Kristensen SL, Rørth R, Jhund PS, et al. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. The lancet Diabetes & endocrinology. 2019;7(10):776-785.
Barkas F, Elisaf M, Milionis H. Protection against stroke with glucagon-like peptide 1 receptor agonists: a systematic review and meta-analysis. European Journal of Neurology. 2019;26(4):559-565.
Bethel MA, Patel RA, Merrill P, et al. Cardiovascular outcomes with glucagon-like peptide-1 receptor agonists in patients with type 2 diabetes: a meta-analysis. The Lancet Diabetes & Endocrinology. 2018;6(2):105-113.
Zelniker TA, Wiviott SD, Raz I, et al. Comparison of the Effects of Glucagon-Like Peptide Receptor Agonists and Sodium-Glucose Cotransporter 2 Inhibitors for Prevention of Major Adverse Cardiovascular and Renal Outcomes in Type 2 Diabetes Mellitus. Circulation. 2019;139(17):2022-2031.
Zheng SL, Roddick AJ, Aghar-Jaffar R, et al. Association Between Use of Sodium-Glucose Cotransporter 2 Inhibitors, Glucagon-like Peptide 1 Agonists, and Dipeptidyl Peptidase 4 Inhibitors With All-Cause Mortality in Patients With Type 2 Diabetes: A Systematic Review and Meta-analysis. Jama. 2018;319(15):1580-1591.
Storgaard H, Cold F, Gluud LL, Vilsbøll T, Knop FK. Glucagon-like peptide-1 receptor agonists and risk of acute pancreatitis in patients with type 2 diabetes. Diabetes, Obesity & Metabolism. 2017;19(6):906-908.
Maiorino MI, Chiodini P, Bellastella G, Capuano A, Esposito K, Giugliano D. Insulin and Glucagon-Like Peptide 1 Receptor Agonist Combination Therapy in Type 2 Diabetes: A Systematic Review and Meta-analysis of Randomized Controlled Trials. Diabetes care. 2017;40(4):614-624.
Maruthur NM, Tseng E, Hutfless S, et al. Diabetes Medications as Monotherapy or Metformin-Based Combination Therapy for Type 2 Diabetes: A Systematic Review and Meta-analysis. Ann Intern Med. 2016;164(11):740-751.
Witkowski M, Wilkinson L, Webb N, Weids A, Glah D, Vrazic H. A Systematic Literature Review and Network Meta-Analysis Comparing Once-Weekly Semaglutide with Other GLP-1 Receptor Agonists in Patients with Type 2 Diabetes Previously Receiving 1-2 Oral Anti-Diabetic Drugs. Diabetes therapy : research, treatment and education of diabetes and related disorders. 2018;9(3):1149-1167.
Levin PA, Nguyen H, Wittbrodt ET, Kim SC. Glucagon-like peptide-1 receptor agonists: a systematic review of comparative effectiveness research. Diabetes, Metabolic Syndrome and Obesity : Targets and Therapy. 2017;10:123-139.
Maciel MG, Beserra BTS, Oliveira FCB, et al. The effect of glucagon-like peptide 1 and glucagon-like peptide 1 receptor agonists on energy expenditure: A systematic review and meta-analysis. Diabetes Research and Clinical Practice. 2018;142:222-235.
Tsapas A, Avgerinos I, Karagiannis T, et al. Comparative Effectiveness of Glucose-Lowering Drugs for Type 2 Diabetes. Annals of internal medicine. 2020;173(4):278-286.
Bassler D, Briel M, Montori VM, et al. Stopping Randomized Trials Early for Benefit and Estimation of Treatment Effects: Systematic Review and Meta-regression Analysis. Jama. 2010;303(12):1180-1187.
Dumville JC, Torgerson DJ, Hewitt CE. Reporting attrition in randomised controlled trials. BMJ (Clinical research ed). 2006;332(7547):969-971.
Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097.
Meta-analysis quantifying effects of GLP1 receptor agonists and SGLT2 inhibitors on cardiovascular risk factors, microvascular and macrovascular outcomes, adverse events, and mortality. PROSPERO; 2018. https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42018090506.
Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ (Clinical research ed). 2011;343:d5928.
Dumville JC, Torgerson DJ, Hewitt CE. Reporting attrition in randomised controlled trials. BMJ (Clinical Research Ed). 2006;332(7547):969-971.
Nakagawa S, Cuthill IC. Effect size, confidence interval and statistical significance: a practical guide for biologists. Biol Rev Camb Philos Soc. 2007;82(4):591-605.
Sterne JA, Sutton AJ, Ioannidis JP, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ (Clinical research ed). 2011;343:d4002.
Morton SC, Murad MH, O’Connor E, et al. AHRQ Methods for Effective Health Care Quantitative Synthesis—An Update. In: Methods Guide for Effectiveness and Comparative Effectiveness Reviews. Rockville (MD): Agency for Healthcare Research and Quality (US); 2008.
Honigberg MC, Chang L-S, McGuire DK, Plutzky J, Aroda VR, Vaduganathan M. Use of Glucagon-Like Peptide-1 Receptor Agonists in Patients With Type 2 Diabetes and Cardiovascular Disease: A Review. JAMA Cardiol. 2020.
Heisler M, Vijan S, Anderson RM, Ubel PA, Bernstein SJ, Hofer TP. When do patients and their physicians agree on diabetes treatment goals and strategies, and what difference does it make? Journal of General Internal Medicine. 2003;18(11):893-902.
Acknowledgements
Contributors: J.A. is the guarantor of this work and had access to the data, designed the study, analyzed and interpreted the data, and drafted the manuscript. N.L. had access to the data, designed the study, analyzed and interpreted data, critically reviewed/edited the manuscript, and obtained funding for the study. E.S., W.W., M.F., A.K., and M.R.S. had access to the data, contributed to the design, analyzed and interpreted data, and reviewed/edited the manuscript. S.B., N.M., E.H., L.P., A.W., C.T., M.Z., V.P., and E.T. contributed to the design and data interpretation, and reviewed/edited the manuscript. K.G., S.J., and B.B. contributed to the design and critically reviewed/edited the manuscript.
Funding
N.L. is supported by the American Diabetes Association (1-18-JDF-037). N.L., E.H., W.W., E.S., and M.F. are members of the NIDDK Chicago Center for Diabetes Translation Research (CCDTR) at the University of Chicago (P30 DK092949).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they do not have a conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 647 kb)
Rights and permissions
About this article
Cite this article
Alexander, J.T., Staab, E.M., Wan, W. et al. The Longer-Term Benefits and Harms of Glucagon-Like Peptide-1 Receptor Agonists: a Systematic Review and Meta-Analysis. J GEN INTERN MED 37, 415–438 (2022). https://doi.org/10.1007/s11606-021-07105-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11606-021-07105-9