Abstract
Background
Atrial fibrillation (AF) is a leading cause of cardiovascular morbidity and mortality. While neighborhood-level factors, such as poverty, have been related to prevalence of AF risk factors, the association between neighborhood poverty and incident AF has been limited.
Objective
Using a large cohort from a health system serving the greater Chicago area, we sought to determine the association between neighborhood-level poverty and incident AF.
Design
Retrospective cohort study.
Participants
Adults, aged 30 to 80 years, without baseline cardiovascular disease from January 1, 2005, to December 31, 2018.
Main Measures
We geocoded and matched residential addresses of all eligible patients to census-level poverty estimates from the American Community Survey. Neighborhood-level poverty (low, intermediate, and high) was defined as the proportion of residents in the census tract living below the federal poverty threshold. We used generalized linear mixed effects models with a logit link function to examine the association between neighborhood poverty and incident AF, adjusting for patient demographic and clinical AF risk factors.
Key Results
Among 28,858 in the cohort, patients in the high poverty group were more often non-Hispanic Black or Hispanic and had higher rates of AF risk factors. Over 5 years of follow-up, 971 (3.4%) patients developed incident AF. Of these, 502 (51.7%) were in the low poverty, 327 (33.7%) in the intermediate poverty, and 142 (14.6%) in the high poverty group. The adjusted odds ratio (aOR) of AF was higher for the intermediate poverty compared with that for the low poverty group (aOR 1.23 [95% CI 1.01–1.48]). The point estimate for the aOR of AF incidence was similar, but not statistically significant, for the high poverty compared with the low poverty group (aOR 1.25 [95% CI 0.98–1.59]).
Conclusion
In adults without baseline cardiovascular disease managed in a large, integrated health system, intermediate neighborhood poverty was significantly associated with incident AF. Understanding neighborhood-level drivers of AF disparities will help achieve equitable care.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Atrial fibrillation (AF) is the most common heart rhythm disorder in US adults and is projected to affect nearly 12 million individuals by the year 2030 [1]. Atrial fibrillation is associated with a five-fold higher risk of stroke [2] and two-fold higher risk of cardiovascular mortality [3]. In addition, atrial fibrillation contributes to rising medical costs on a population level [4] as well as poorer patient-reported quality of life on an individual level [5].
Prior evidence has identified several clinical risk factors associated with the development of atrial fibrillation, including obesity, diabetes, and hypertension [6]. However, as increasing attention is paid to the social determinants of cardiovascular disease, it is uncertain how sociodemographic factors influence the risk of developing atrial fibrillation [7]. Whereas prior studies have examined the relationship between individual-level socioeconomic status (e.g., race, ethnicity) and atrial fibrillation–related treatment and outcomes [8, 9], few have specifically assessed the association of socioeconomic factors with the onset of atrial fibrillation [10, 11]. Previous studies have demonstrated an association between neighborhood-level deprivation and multiple clinical risk factors for developing atrial fibrillation, suggesting the factors driving this deprivation may contribute to the incidence burden of atrial fibrillation [12,13,14,15]. Yet, understanding poverty as a social determinant of health and key component of neighborhood deprivation, through its influence either on the built environment or on the persistence of clinical risk factors, remains an important gap in the atrial fibrillation literature that requires direct examination. This is especially important in a diverse, urban patient population residing in high poverty neighborhoods.
Using data from a large integrated health system that serves the greater Chicago area, we retrospectively examined the association between neighborhood-level poverty and incidence of atrial fibrillation in a cohort of adults without baseline cardiovascular disease.
METHODS
Data Sources
The Northwestern Medicine Enterprise Data Warehouse (NMEDW) was created in 2007 and serves as an electronic health repository of data from an integrated health system with over 200 primary clinical sites in the metropolitan Chicago area [16]. The NMEDW stores observations on over 6 million patients, loading nearly 3 billion new data elements from 142 separate sources each day. The NMEDW includes individuals receiving care at any of the Northwestern Medicine–affiliated institutions, comprising tertiary and secondary hospitals and inpatient and outpatient sites throughout northeastern Illinois [16].
The data for all neighborhood-level factors in this analysis were ascertained using the 2007–2011 American Community Survey (ACS) [17, 18]. The ACS is a repeated, cross-sectional survey conducted by the US Census Bureau administered annually to a nationally representative sample of over 3 million American households [19]. The survey collects social and economic characteristics of persons and households such as age, gender, race, income, education, and employment. The additional collection of Federal Information Processing Standards codes permits the survey data to be linked with other databases at the state, county, and census tract levels [20].
Study Population
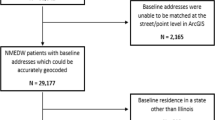
The study population consisted of patients in the NMEDW who were 30–80 years old at the time of their first outpatient visit in the health system between January 1, 2005, and December 31, 2013. Patients were included if they were free of cardiovascular disease at this index outpatient visit (i.e., baseline), and if they had at least 5 years of follow-up (through December 31, 2018). For those with historical records, a look-back period prior to January 1, 2005, was also performed to confirm no prior history of cardiovascular disease, defined by a diagnosis of atrial fibrillation, coronary artery disease, stroke/cerebrovascular disease, peripheral arterial disease, or heart failure, or presence of a pacemaker prior to the baseline visit (Appendix Table 1). Additionally, patients were excluded if missing measurements for key covariates at baseline including smoking status, body mass index (BMI), blood pressure, and glucose levels. Billing addresses from the baseline visit were geocoded using ArcGIS Pro and matched to ACS census tract–level poverty estimates. Patients were excluded from the analysis if their addresses could not be accurately geocoded (N = 2165) or if their baseline address was outside of Illinois (N = 319) resulting in a final study population of 28,858 patients (Fig. 1).
Primary Exposure and Outcome Ascertainment
Neighborhood-level poverty is the primary exposure for this analysis. The census tract is used as a proxy for neighborhood, as previously described [21]. Neighborhood poverty is quantified as the proportion of people in a given census tract living below the US-defined poverty threshold (income to poverty ratio less than 1) [22]. Using all of the available census tract–level poverty estimates for Illinois, we calculated tertiles of this proportion to derive three categories of neighborhood poverty (Fig. 2): low poverty (proportion below poverty threshold less than 0.073, which translates to approximately 7% of the census tract living below the US-defined poverty threshold), intermediate poverty (proportion below poverty threshold from 0.073 to 0.16, or between 7 and 16% of households living below the poverty threshold), and high poverty (proportion below poverty threshold greater than 0.16, or greater than 16% of households living below the poverty threshold) [22, 23].
Map of poverty categories for the greater Chicago area. Shading of census tracts corresponds to poverty category with the lightest blue representing neighborhoods with the lowest proportion of residents below the US-defined poverty level and the darkest blue representing neighborhoods with the highest proportion of residents above the US-defined poverty level.
The primary outcome is incidence of atrial fibrillation in the follow-up period. Atrial fibrillation was defined as the first atrial fibrillation diagnosis (inpatient or outpatient) using previously established ICD-9-CM (427.31) and ICD-10-CM (I48.0, I48.1, I48.2, I48.9, I48.91) diagnosis codes for atrial fibrillation [8].
Baseline Covariates
Additional covariates of interest included baseline demographics and other known clinical risk factors for atrial fibrillation. Baseline demographics included age at baseline visit, sex, and race/ethnicity. Race/ethnicity was categorized as non-Hispanic White, non-Hispanic Black, Hispanic, and other. Clinical risk factors for atrial fibrillation included objectively assessed body mass index (calculated using height and weight measures) and systolic and diastolic blood pressure. Laboratory testing included random glucose levels. Hypertension was defined as having a systolic blood pressure of 140 mmHg or higher, anti-hypertensive medication use, or a hypertension diagnosis per ICD-9 and 10 diagnosis codes. Diabetes was defined as having a hemoglobin A1C level of 6.5% or higher, anti-diabetic medication use, or a diabetes diagnosis per ICD codes. Obesity was defined by BMI ≥ 30 kg/m2. Smoking status was defined as being a current smoker (every day or some days) or not a current smoker. Diagnosis codes used for clinical risk factor identification can be found in Appendix Table 1.
Statistical Analysis
We compared baseline demographic and clinical characteristics across tertiles of neighborhood poverty using chi-square tests for categorical variables and analysis of variance or Kruskal-Wallis tests for continuous variables. Generalized linear mixed effects models with a logit link function were used to evaluate the association between neighborhood-level poverty and incident atrial fibrillation while accounting for the non-independence of patients residing within the same census tract using a random intercept for census tract. We accounted for the non-independence of patients residing within the same neighborhood through the inclusion of a random intercept for census tract. The relationship between neighborhood poverty category and atrial fibrillation was assessed using a series of sequentially adjusted models: unadjusted (Model 1), adjusted for demographics including continuous age, sex, and race/ethnicity (Model 2), and additionally adjusted for clinical risk factors, including smoking, continuous BMI, hypertension, and diabetes (Model 3). We examined whether the relationship between neighborhood-level poverty and atrial fibrillation differed by race/ethnicity by testing an interaction term in the fully adjusted model and observed that this interaction was not statistically significant (p >0.05); thus, these findings are not included in the results below.
We used a two-tailed p value of < 0.05 to define statistical significance. All statistical analyses were performed using R version 3.6.1 or SAS version 9.4. This study was approved by the Northwestern University Feinberg School of Medicine Institutional Review Board.
RESULTS
Baseline Characteristics
The final cohort comprised 28,858 patients, including 15,952 (55.3%) that resided in a neighborhood characterized as having a low proportion of residents living below the poverty threshold (i.e., low poverty tertile), 8474 (29.4%) that resided in an intermediate poverty neighborhood, and 4432 (15.4%) that resided in a neighborhood with a high proportion of residents living below the federal poverty level (i.e., high poverty tertile) (Table 1). The mean age (standard deviation) of individuals in the cohort was 51.4 (±11.3) years and included 2994 (10.4%) non-Hispanic Black and 1397 (4.8%) Hispanic patients as well as 16,578 (57.4%) women. Patients living in high poverty neighborhoods were more often non-Hispanic Black or Hispanic and current smokers, and had higher rates of obesity, hypertension, and diabetes.
Incidence of Atrial Fibrillation
At the 5-year follow-up, 971 (3.4%) patients developed atrial fibrillation. Of those who developed atrial fibrillation, 502 (51.7%) were in the low poverty, 327 (33.7%) in the intermediate poverty, and 142 (14.6%) in the high poverty group (Table 1). Women, non-Hispanic Black individuals, and those who idenfied their race as other had lower odds of developing atrial fibrillation in adjusted analyses. Atrial fibrillation risk factors, including BMI and hypertension, were associated with greater incidence of atrial fibrillation. The unadjusted odds of developing atrial fibrillation in the intermediate poverty group (odds ratio, OR 1.13 [95% CI 0.94–1.35]) and high poverty group (OR 0.96 [95% CI 0.78–1.20]) were not significantly different compared to the low poverty group (Table 2). When adjusting for sociodemographic factors, patients in the intermediate poverty group had a higher incidence of atrial fibrillation compared with the low poverty group (OR 1.21 ([95% CI 1.00–1.46]). The point estimate for the odds ratio was similar between the high versus low poverty groups but was not statistically significant (OR 1.23 [95% CI 0.96, 1.57]). When adjusting further for clinical risk factors, patients in the intermediate poverty group still had a higher incidence of atrial fibrillation compared with the low poverty group (OR 1.23 [95% CI 1.01–1.48]). The point estimate for the fully adjusted odds ratio was similar when comparing the high and low poverty groups (OR 1.25 [95% CI 0.98, 1.59]), but was not significantly different (Table 2).
DISCUSSION
Atrial fibrillation is an increasingly common and morbid heart rhythm disorder with substantial medical and societal costs [24]. In this study, we examined a real-world, retrospective cohort of adults free of cardiovascular disease at baseline and observed an association between intermediate neighborhood-level poverty and incident atrial fibrillation after adjusting for demographic and clinical risk factors, with a trend toward significance for high neighborhood-level poverty, though limited by the relatively small size of the high poverty group. Identifying factors, such as neighborhood-level poverty, beyond the traditional clinical risk factors associated with atrial fibrillation may be useful to ensuring equitable prevention of this condition.
Results in Context of Individual Versus Neighborhood Socioeconomic Status
Prior studies have examined the association between individual- or household-level socioeconomic status (SES) and incidence of atrial fibrillation [8, 9]. Two analyses of the Atherosclerosis Risk in Communities (ARIC) study in 2014 and 2018 reported inverse relationships between individual-level SES and atrial fibrillation. The cumulative incidence of atrial fibrillation was lower among those with higher SES (i.e., self-reported family income and education level) independent of traditional atrial fibrillation risk factors in a prospective cohort of over 14,000 patients recruited from 1987 to 1989 [10, 25]. Examining broader neighborhood-level SES and utilizing a contemporary, more racially and ethnically diverse patient cohort, the current analysis also observed an inverse relationship, although the association between neighborhoods with the highest proportion of residents living below the US-defined poverty level and incident atrial fibrillation was not statistically significant.
Two additional reports out of Sweden [26] and New York City [27] examined the association between combined measures of neighborhood-level SES (e.g., factors such as neighborhood-level median household income, education level, employment status, and receipt of social services) and incident atrial fibrillation. Respectively, the Swedish study found an inverse association between SES and hospitalization for atrial fibrillation (in women, but not in men), while the New York City study did not observe a statistically significant relationship between a composite SES score and incident atrial fibrillation. These contradictory findings reveal the complex interplay between neighborhood SES and incidence of atrial fibrillation. The disparate findings, compared with the current analysis, may reflect patient-level demographic and clinical differences in the two cohorts, time lapse between SES measurement and the diagnosis of atrial fibrillation, and limitations in atrial fibrillation ascertainment in the previous analyses, including the use of electrocardiograms only without atrial fibrillation diagnosis code assessment in the New York City study.
Determinants of Neighborhood-Level Poverty and Atrial Fibrillation Incidence
The relationship between neighborhood-level poverty and atrial fibrillation incidence is complex and is likely confounded by the relationship between race/ethnicity and the structural environment. As observed in our analysis, individuals residing in higher poverty neighborhoods are more likely to be non-Hispanic Black or Hispanic [28] and have a higher rate of clinical risk factors for atrial fibrillation including smoking, obesity, hypertension, and diabetes. Factors present in poorer neighborhoods such as decreased access to healthy foods, limited green space, and scarce public safety resources to support physical activity likely exacerbate these clinical risk factors [13]. Other determinants of our findings may include the fact that neighborhoods with a greater proportion of residents living below the US-defined poverty level tend to have higher rates of air pollution [29]. Air pollution is a known risk factor broadly associated with cardiovascular disease [29], and an increasingly recognized risk factor for atrial fibrillation, which may influence atrial fibrillation incidence in high poverty communities [30, 31]. Additionally, increased allostatic load, a concept related to physiologic stress, has been reported in individuals residing in high poverty compared with low poverty neighborhoods and has also been connected with the development of cardiovascular disease [32]. These factors may explain higher incidence of atrial fibrillation in higher poverty groups though they were not directly examined in our analysis and would further not explain why the association between high poverty and incident atrial fibrillation was not statistically significant.
In contrast, there are characteristics of individuals residing in high poverty neighborhoods that have been associated with lower incidence of atrial fibrillation. First, racial/ethnic differences in atrial fibrillation incidence have been previously described, with Black and Hispanic individuals demonstrating a lower risk of atrial fibrillation compared with their White counterparts [33]. These racial/ethnic differences have been largely attributed to genetic factors, with a greater incidence of atrial fibrillation reported in individuals of European ancestry [34, 35]. However, decreased geographic or insurance-related access to or limited engagement in health care, decreased access to cardiovascular specialist care, and provider bias for individuals residing in high poverty neighborhoods may result in lower ascertainment of atrial fibrillation in these communities [36]. Finally, it is well-established that life expectancy is reduced among lower income adults on the individual and population level [37, 38]. Thus, a higher rate of competing risk of premature death in high compared with low poverty neighborhoods [39] may result in fewer individuals in the highest poverty neighborhoods developing atrial fibrillation over time, given the significant association between aging and atrial fibrillation incidence [2]. This observation represents an important area of future exploration in the study of the relationship between poverty and incident atrial fibrillation.
Strengths and Limitations
This study has several strengths. The data were obtained from a large retrospective cohort with detailed sociodemographic and comprehensive clinical risk factor data. The analysis used robust geocoding methodology along with census tract–level data to assess neighborhood poverty in a representative, diverse population.
There are also certain limitations. First, because this was an observational study, we cannot exclude the possibility of residual or unmeasured confounding, particularly in our assessment of individual-level social risk factors for atrial fibrillation, including education, insurance, and employment status or other neighborhood-level factors such as environment exposures, racial composition, or community-level access to care. Second, while we adjusted for clinical risk factor burden at baseline, we were not able to capture cumulative burden over time, which has been associated with adverse cardiovascular remodeling and events that may predispose to atrial fibrillation. Third, as cohort entry required individuals to have at least 5 years of follow-up data as well as to have a matchable address, we cannot exclude the possibility of selection bias due to loss to follow-up. Fourth, our ascertainment of atrial fibrillation comprised using ICD codes alone and did not include electrocardiographic or Holter monitoring data, nor whether an atrial fibrillation diagnosis was paroxysmal or asymptomatic, which may have resulted in under-ascertainment of atrial fibrillation cases. While this method makes it difficult to capture the true burden of atrial fibrillation, it is likely we were able to adequately capture a large proportion of symptomatic atrial fibrillation that required interaction with the healthcare system. Furthermore, our rates of incident atrial fibrillation were similar to prior population-based cohort studies (e.g., the Multi-Ethnic Study of Atherosclerosis) that incorporated independent physician-based event adjudication and electrocardiographic data [40]. Finally, our analysis did not include an examination of possible clustering of potential neighborhood-specific atrial fibrillation–related exposures, an important area of future study.
CONCLUSION
In a large, diverse, real-world cohort of patients without baseline cardiovascular disease, we found that individuals living in the neighborhoods at the intermediate poverty level had a higher incidence of atrial fibrillation over time with a trend toward a higher incidence in individuals residing in neighborhoods at the highest poverty level. Understanding how neighborhood- and individual-level clinical and sociodemographic factors interact to increase the risk of atrial fibrillation is critical to developing equitable prevention strategies in this increasingly common and morbid cardiovascular condition.
References
Benjamin EJ, Muntner P, Alonso A, et al. Heart Disease and Stroke Statistics-2019 Update: a Report From the American Heart Association. Circulation. 2019. doi:https://doi.org/10.1161/CIR.0000000000000659
Wolf PA, D’Agostino RB, Belanger AJ, Kannel WB. Probability of Stroke: a Risk Profile from the Framingham Study. Stroke. 1991;22(3):312-318. doi:https://doi.org/10.1161/01.STR.22.3.312
Benjamin EJ, Wolf PA, D’Agostino RB, Silbershatz H, Kannel WB, Levy D. Impact of Atrial Fibrillation on the Risk of Death: the Framingham Heart Study. Circulation. 1998. doi:https://doi.org/10.1161/01.CIR.98.10.946
Kim MH, Johnston SS, Chu B, Dalal MR, Schulman KL. Estimation of Total Incremental Health Care Costs in Patients with Atrial Fibrillation in the United States. Circ Cardiovasc Qual Outcomes. 2011;4:313-320. doi:https://doi.org/10.1161/CIRCOUTCOMES.110.958165
Golwala H, Jackson LR, Simon DN, et al. Racial/Ethnic Differences in Atrial Fibrillation Symptoms, Treatment Patterns, and Outcomes: Insights from Outcomes Registry for Better Informed Treatment for Atrial Fibrillation Registry (ORBIT-AF). Am Heart J. 2016;174:29-36. doi:https://doi.org/10.1016/j.ahj.2015.10.028
Mann I, Sandler B, Linton N, Kanagaratnam P. Drivers of Atrial Fibrillation: Theoretical Considerations and Practical Concerns. Arrhythmia Electrophysiol Rev. 2018. doi:https://doi.org/10.15420/aer.2017.40.3
Essien UR, Kornej J, Johnson AE, Schulson LB, Benjamin EJ, Magnani JW. Social determinants of atrial fibrillation. Nat Rev Cardiol. 2021 Jun 2. https://doi.org/10.1038/s41569-021-00561-0
Essien UR, Holmes DN, Jackson LR, et al. Association of Race/Ethnicity with Oral Anticoagulant Use in Patients with Atrial Fibrillation. JAMA Cardiol. 2018. doi:https://doi.org/10.1001/jamacardio.2018.3945
Carlsson AC, Wändell P, Gasevic D, Sundquist J, Sundquist K. Neighborhood Deprivation and Warfarin, Aspirin and Statin Prescription — a Cohort Study of Men and Women Treated for Atrial Fibrillation in Swedish primary care. Int J Cardiol. 2015;187:547-552. doi:https://doi.org/10.1016/j.ijcard.2015.04.005
Misialek JR, Rose KM, Everson-Rose SA, et al. Socioeconomic Status and the Incidence of Atrial Fibrillation in Whites and Blacks: the Atherosclerosis Risk in Communities (ARIC) Study. J Am Heart Assoc. 2014. doi:https://doi.org/10.1161/JAHA.114.001159
Shulman E, Chudow JJ, Essien UR, et al. Relative Contribution of Modifiable Risk Factors for Incident Atrial Fibrillation in Hispanics, African Americans and non-Hispanic Whites. Int J Cardiol. 2019. doi:https://doi.org/10.1016/j.ijcard.2018.10.028
Mayne SL, Moore KA, Powell-Wiley TM, Evenson KR, Block R, Kershaw KN. Longitudinal Associations of Neighborhood Crime and Perceived Safety with Blood Pressure: the Multi-Ethnic Study of Atherosclerosis (MESA). Am J Hypertens. 2018. doi:https://doi.org/10.1093/ajh/hpy066
Kershaw KN, Robinson WR, Gordon-Larsen P, et al. Association of Changes in Neighborhood-Level Racial Residential Segregation with Changes in Blood Pressure Among Black Adults: the CARDIA study. JAMA Intern Med. 2017. doi:https://doi.org/10.1001/jamainternmed.2017.1226
Bancks MP, Kershaw K, Carson AP, Gordon-Larsen P, Schreiner PJ, Carnethon MR. Association of Modifiable Risk Factors in Young Adulthood with Racial Disparity in Incident Type 2 Diabetes During Middle Adulthood. JAMA - J Am Med Assoc. 2017. doi:https://doi.org/10.1001/jama.2017.19546
Mayne SL, Jose A, Mo A, et al. Neighborhood Disorder and Obesity-Related Outcomes Among Women in Chicago. Int J Environ Res Public Health. 2018. doi:https://doi.org/10.3390/ijerph15071395
Starren JB, Winter AQ, Lloyd-Jones DM. Enabling a Learning Health System Through a Unified Enterprise Data Warehouse: the Experience of the Northwestern University Clinical and Translational Sciences (NUCATS) Institute. Clin Transl Sci. 2015. doi:https://doi.org/10.1111/cts.12294
Yasenov VI, Lawrence D, Mendoza FS, Hainmueller J. Public Health Insurance Expansion for Immigrant Children and Interstate Migration of Low-Income Immigrants. JAMA Pediatr. 2020. doi:https://doi.org/10.1001/jamapediatrics.2019.4241
U.S. Census Bureau. A compass for understanding and using American Community Survey data: what PUMS data users need to know [Internet]. Washington DC: U.S. Census Bureau; 2009. Available from: https://www.census.gov/content/dam/Census/library/publications/2009/acs/ACSPUMS.pdf
U.S. Census Bureau. American Community Survey. Availabele at: https://www.census.gov/programs-surveys/acs/. Accessed April 12, 2019.
Tung EL, Hampton DA, Kolak M, Rogers SO, Yang JP, Peek ME. Race/Ethnicity and Geographic Access to Urban Trauma Care. JAMA Netw open. 2019. doi:https://doi.org/10.1001/jamanetworkopen.2019.0138
Kolak M, Bhatt J, Park YH, Padrón NA, Molefe A. Quantification of Neighborhood-Level Social Determinants of Health in the Continental United States. JAMA Netw open. 2020. doi:https://doi.org/10.1001/jamanetworkopen.2019.19928
Claudel SE, Adu-Brimpong J, Banks A, et al. Association Between Neighborhood-Level Socioeconomic Deprivation and Incident Hypertension: a Longitudinal Analysis of Data from the Dallas Heart Study. Am Heart J. 2018. doi:https://doi.org/10.1016/j.ahj.2018.07.005
Rethy LB, McCabe ME, Kershaw KN, Ahmad FS, Lagu T, Pool LR, Khan SS. Neighborhood Poverty and Incident Heart Failure: an Analysis of Electronic Health Records from 2005 to 2018. J Gen Intern Med. 2021 May 7. doi: https://doi.org/10.1007/s11606-021-06785-7.
Anter E, Jessup M, Callans DJ. Atrial Fibrillation and Heart Failure: Treatment Considerations for a Dual Epidemic. Circulation. 2009. doi:https://doi.org/10.1161/CIRCULATIONAHA.108.821306
Mou L, Norby FL, Chen LY, et al. Lifetime Risk of Atrial Fibrillation by Race and Socioeconomic Status. Circ Arrhythmia Electrophysiol. 2018. doi:https://doi.org/10.1161/circep.118.006350
Zöller B, Li X, Sundquist J, Sundquist K. Neighbourhood Deprivation and Hospitalization for Atrial Fibrillation in Sweden. Europace. 2013. doi:https://doi.org/10.1093/europace/eut019
Shulman E, Kargoli F, Aagaard P, et al. Socioeconomic Status and the development of Atrial Fibrillation in Hispanics, African Americans and non-Hispanic Whites. Clin Cardiol. 2017. doi:https://doi.org/10.1002/clc.22732
Skosireva A, O’Campo P, Zerger S, Chambers C, Gapka S, Stergiopoulos V. Different Faces of Discrimination: Perceived Discrimination Among Homeless Adults with Mental Illness in Healthcare Settings. BMC Health Serv Res. 2014. doi:https://doi.org/10.1186/1472-6963-14-376
Hicken MT, Adar SD, Hajat A, et al. Air Pollution, Cardiovascular Outcomes, and Social Disadvantage: the Multi-ethnic Study of Atherosclerosis. Epidemiology. 2016. doi:https://doi.org/10.1097/EDE.0000000000000367
Monrad M, Sajadieh A, Christensen JS, et al. Long-Term Exposure to Traffic-Related Air Pollution and Risk of Incident Atrial Fibrillation: a Cohort Study. Environ Health Perspect. 2017. doi:https://doi.org/10.1289/EHP392
Rhinehart ZJ, Kinnee, E, Essien UR et al. Association of Fine Particulate Matter and Risk of Stroke in Patients with Atrial Fibrillation. JAMA Network Open. 2020;3(9):e2011760. doi:https://doi.org/10.1001/jamanetworkopen.202011760
Wändell P, Carlsson AC, Gasevic D, et al. Socioeconomic Factors and Mortality in Patients with Atrial Fibrillation—a Cohort Study in Swedish Primary Care. Eur J Public Health. 2018. doi:https://doi.org/10.1093/eurpub/cky075
Ugowe FE, Jackson LR, Thomas KL. Racial and Ethnic Differences in the Prevalence, Management, and Outcomes in Patients with Atrial Fibrillation: a Systematic Review. Hear Rhythm. 2018. doi:https://doi.org/10.1016/j.hrthm.2018.05.019
Marcus GM, Alonso A, Peralta CA, et al. European Ancestry as a Risk Factor for Atrial Fibrillation in African Americans. Circulation. 2010. doi:https://doi.org/10.1161/CIRCULATIONAHA.110.958306
Dewland TA, Olgin JE, Vittinghoff E, Marcus GM. Incident Atrial Fibrillation Among Asians, Hispanics, Blacks, and Whites. Circulation. 2013;128(23):2470-2477. doi:https://doi.org/10.1161/CIRCULATIONAHA.113.002449
Meschia JF, Merrill P, Soliman EZ, et al. Racial Disparities in Awareness and Treatment of Atrial Fibrillation: the REasons for Geographic And Racial Differences in Stroke (REGARDS) Study. Stroke. 2010;41(4):581-587. doi:https://doi.org/10.1161/STROKEAHA.109.573907
Dwyer-Lindgren L, Bertozzi-Villa A, Stubbs RW, et al. Inequalities in life Expectancy Among US Counties, 1980 to 2014. JAMA Intern Med. 2017. doi:https://doi.org/10.1001/jamainternmed.2017.0918
Woolf SH, Schoomaker H. Life Expectancy and Mortality Rates in the United States, 1959-2017. JAMA - J Am Med Assoc. 2019. doi:https://doi.org/10.1001/jama.2019.16932
Shah NS, Lloyd-Jones DM, O’Flaherty M, et al. Trends in Cardiometabolic Mortality in the United States, 1999-2017. JAMA. 2019. doi:https://doi.org/10.1001/jama.2019.9161
Alonso A, Roetker NS, Soliman EZ, Chen LY, Greenland P, Heckbert SR. Prediction of Atrial Fibrillation in a Racially Diverse Cohort: the Multi-ethnic Study of Atherosclerosis (mesa). J Am Heart Assoc. 2016. doi:https://doi.org/10.1161/JAHA.115.003077
Funding
The American Heart Association
Author information
Authors and Affiliations
Contributions
The authors thank Terrence M.A. Litam for his assistance in preparing this manuscript.
Corresponding author
Ethics declarations
Ethics Approval
This study was approved by the Northwestern University Feinberg School of Medicine Institutional Review Board.
Conflict of Interest
The authors declare that they do not have a conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 16 kb)
Rights and permissions
About this article
Cite this article
Essien, U.R., McCabe, M.E., Kershaw, K.N. et al. Association Between Neighborhood-Level Poverty and Incident Atrial Fibrillation: a Retrospective Cohort Study. J GEN INTERN MED 37, 1436–1443 (2022). https://doi.org/10.1007/s11606-021-06976-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11606-021-06976-2