Abstract
Background
Osteoarthritis (OA) is common and burdensome for patients and health care systems. Our study purpose was to evaluate the long-term efficacy and safety of DMOADs in adults with knee and hip osteoarthritis.
Methods
We searched Cochrane Central Register of Controlled Trials, MEDLINE, EMBASE, and Web of Knowledge without language, publication, or date restrictions from inception through November 2018 for randomized controlled trials assessing 12 classes of DMOADs with at least 12 months of follow-up. Therapeutic effects were evaluated with pairwise and network meta-analysis. Outcomes included pain, function, minimum joint space width or cartilage volume, radiographic progression, and total joint replacement. Analyses were also performed for drug safety.
Results
Twenty-eight randomized controlled trials with 11,890 patients were included. Glucosamine and chondroitin minimally improved both structure (minimum joint width or cartilage volume: network results: glucosamine: SMD 0.16; 95% CI [0.04, 0.28], chondroitin: SMD 0.21 [0.10, 0.32]) and symptoms (glucosamine: pain: − 0.15 [− 0.25, − 0.05]; function: − 0.17 [− 0.28, − 0.07], chondroitin: pain: − 0.06 [− 0.15, 0.03], and function: − 0.15 [− 0.26, − 0.03]). Strontium demonstrated improvement in structure (minimum joint width or cartilage volume: 0.20 [0.02, 0.38]), and vitamin D on symptoms (pain: − 0.15 [− 0.27, -0.03]; function: − 0.18 [− 0.31, − 0.06]). Although doxycycline also demonstrated a favorable efficacy ranking, its safety profile was poor (withdrawal: network relative risk 1.69 [1.03, 2.75]). The therapeutic effects of other medications were not ranked as highly.
Discussion
Glucosamine and chondroitin yielded statistically significant but clinically questionable long-term benefit on structure and symptoms, though both had favorable safety profiles. Strontium improved structure, and vitamin D improved symptoms. Although doxycycline had a favorable efficacy ranking, its safety profile was poor. None of the 12 classes of drugs appears to have long-term clinically significant benefit.
Similar content being viewed by others
INTRODUCTION
Osteoarthritis is a major contributor to chronic pain and physical disabilities globally.1, 2 Current pharmaceutical treatment for OA is largely restricted to analgesics including non-steroidal anti-inflammatory drugs (NSAIDs), which are both palliative in nature and accompanied by adverse effects.3 Fortunately, an array of etiologically targeted agents, known as disease-modifying OA drugs, have been developed in the hope of slowing its progression and provide symptomatic benefits4 (Appendix 1.1). These drugs act via a variety of mechanisms and targets including subchondral bone, cartilage, and synovium. While well-known potential DMOADs, such as glucosamine and chondroitin, are popular and used worldwide, they remain controversial in official recommendations and meta-analytic conclusions.5–8 Although there have been increasingly emerging potential disease-modifying drugs for OA, many unanswered questions persist regarding their treatment efficacy as well as underlying physiological mechanism and mode of action.
The aim of our study was to compare the long-term efficacy and safety of potential DMOADs on hip and knee OA.9
METHODS
Search Strategy and Selection Criteria
We included randomized placebo-controlled and comparative efficacy trials that evaluated orally administered DMOADs for knee or hip OA. Additional inclusion criteria included the duration of treatment was at least 12 months (6 months for MRI10) and included at least one structural outcome. We excluded hand OA patients because they are non-weight-bearing joints. There is evidence that OA at different joint types bears distinguishable pathophysiological mechanisms due to different anatomy and physiology and may respond differently to pharmacologic interventions.11–13 We included the following candidate drugs14, 15: chondroitin, glucosamine, diacerein, matrix metalloproteinase (MMP) inhibitors, collagen hydrolysate, vitamin E, vitamin D, inducible nitric oxide synthase (iNOS) inhibitors, doxycycline, avocado-soybean unsaponifiables (ASUs), hyaluronic acid, bisphosphonates, strontium ranelate, calcitonin, and licofelone.
The Cochrane Central Register of Controlled Trials, MEDLINE, EMBASE, and Web of Knowledge were searched without language, publication, or date restrictions on our own.16 A representative search strategy (Appendix 1.2) was further modified for grey literature. Reference lists from selected publications were screened by three reviewers for additional studies. The same three reviewers independently examined the article abstracts, and upon selection, full-text reports were evaluated for final inclusion.
Data Analysis
Data were extracted independently by two reviewers using a predesigned standardized form. Attempts were made to contact the authors to supplement incomplete reports; for those failing to respond, means and variance were estimated from available text and figures.14–19 Changes from baseline values were used to assess continuous outcomes,16 but if unreported, these values were derived from the baseline and final measurements14, 20–27 (Appendix 1.5, with correlation coefficients for standard deviation (SD) set as 0.528). Wherever possible, the results from the intention to treat (ITT) analysis were used.
The quality of evidence was assessed following the GRADE system.29 Study limitations were described in the risk of bias tables, plots, and contribution matrices.16 Common heterogeneity (Tau2) was assumed across all treatment comparisons in the NMA,30 while 95% confidence and prediction intervals of the NMA estimate were applied to assess the magnitude of heterogeneity.31 Consistency was tested both globally (design by treatment inconsistency model) and locally (side-splitting and loop-specific approach), with significance set at p <0.1.30 NMA transitivity was evaluated to assess the indirectness of studies. Finally, sensitivity analyses were undertaken for subgroups (including joint types, doses, dosing schedules, and product subtypes) and publication bias (using funnel plots, and analyses after excluding outliers).
Joint space narrowing on radiograph remains the only validated primary endpoint to assess the structural progression of OA and is recommended by both professional societies and regulatory agencies in the USA and Europe.17–19 The predefined primary outcome was therefore the structural efficacy on the minimum joint space width (weight-bearing X-ray). The relatively new cartilage volume measurement by MRI was also accepted in the analysis.20–22 Radiographic progression and total joint replacement were also included as adjunct structural outcomes. Changes in pain and function were analyzed as symptom outcomes, and if a study utilized more than one pain or function scale, then the result for the highest scale on the hierarchy list was extracted.23, 24 To assess safety profiles, drug-specific adverse events and study withdrawal rates were analyzed.
Wherever possible, data from primary studies were summarised in the meta-analysis, and if not, they were discussed in a narrative manner. Continuous variables were described with standardized mean differences (SMDs) using 95% confidence intervals (CI), while relative risk (RR) with a 95% CI was used to report dichotomous data. SMDs greater than 0.8 and RR greater than 2 or less than 0.5 were defined as a large effect, SMDs between 0.5 and 0.8 and RR between 1.25 and 2 or 0.5 and 0.8 were defined as a moderate effect, and SMDs of less than 0.5 and RR between 1 and 1.25 or 0.8 and 1 were defined as a small effect. Clinical effectiveness was defined as at least with a moderate or large SMD.25, 26, 58 The combination at different time points from a single study was performed following formulations in Appendix 1.5 (correlation coefficients for SD set as 1.0).16, 27 For those trials reporting multiple intervention groups (e.g., different dosages) with a common control group, groups were combined based on the formulations in Appendix 1.5.16, 27
In the pairwise meta-analysis, random-effects modeling was employed. The number needed to harm (NNH) was calculated for adverse events. Forest plots were employed to compare the treatment effects of each candidate. A NMA under a frequentist framework was introduced to optimize direct and indirect evidence and to rank all competing DMOAD candidates. In the NMA, random-effects modeling was adopted. The geometry and patterns of the network were prepared for each outcome, and the efficacy/safety hierarchy for a specific outcome was obtained via the surface under the cumulative ranking curve (SUCRA).28 Clustered ranking plots were based on the cluster analysis of SUCRA values for two outcomes and displayed using the two dimensions of the x- and y-axes.28
Analyses were performed using RevMan (version 5.3, Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2015), Confidence in Network Meta-Analysis (University of Bern 2017, available from cinema.ispm.ch), and STATA (version 14, STATA cooperation, USA, 2015). P values were two-sided with α = 0.05. Patients or the public were not involved in the design, or conduct, or reporting, or dissemination of our research.
RESULTS
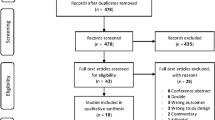
After application of inclusion and exclusion criteria (Fig. 1), 30 records (28 studies) were included.21, 22, 29–56 Overall, 7356 patients were randomized to treatment with a DMOAD, and 4534 were randomized to comparator groups, with well-balanced baseline characteristics (Appendix 1.3). Although a small-scale trial of collagen hydrolysate was identified,25 it was excluded due to the cartilage volume measurement approach. Hyaluronic acid was excluded based on its administration route. Details of the included trials are listed in Appendix 1.3. The network geometry (Fig. 2 and Appendices 2.2.4, 2.3.4, 3.1.4.3.2.4) revealed a star-shaped network with multi-arm trials (Appendices 2.1.3, 2.2.5, 2.3.5, 3.1.5, 3.2.5, 4.3.1). For our primary structural outcomes of minimum joint space or cartilage volume, the pairwise meta-analysis (Fig. 3) revealed significant effects at combined time points for chondroitin, doxycycline, and strontium ranelate. The network ranking (Appendix 2.1.4, 2.1.5 and Fig. 4) verified statistical significance but demonstrated low clinical meaningfulness of chondroitin (network SMD 0.21; CI [0.10, 0.32]; SUCRA 78.1; rank 1), strontium (0.20; [0.02, 0.38]; 74.4; 2), and glucosamine (0.16; [0.04, 0.28]; 65.2; 6). There was a trend towards greater improvement from 1 to 3 years for most medications (Appendix 2.1.1 and 2.1.2). In terms of radiographic progression, a pairwise meta-analysis identified strontium and diacerein as statistically effective agents (Appendix 2.2.1, 2.2.2, and 2.2.3), although the NMA indicated that chondroitin (network RR 0.59; CI [0.45, 0.77]; SUCRA 93.2; rank 1) and glucosamine (0.62; [0.45, 0.86]; 88.0; 2) were better (Appendix 2.2.6, 2.2.7, and 2.2.8). Seven studies31, 36, 37, 42, 51, 53, 56 reported the outcome of total joint replacement and the pooled estimate effects were insignificant in either the pairwise (Appendix 2.3.1, 2.3.2, and 2.3.3) or the NMA for any of the medications (Appendix 2.3.6, 2.3.7, and 2.3.8).
Network maps of DMOADs regarding minimum JSW or cartilage volume (a) and withdrawals due to adverse events (b). The width of lines is proportional to the number of studies compared in every pair of treatments, and the size of nodes is proportional to the total sample size of each treatment. * licofelone study adopted naproxen instead of a placebo as a comparator. ASU, avocado soy unsaponifiables; DMOADs, disease-modifying osteoarthritis drugs; iNOS, inducible nitric oxide synthase; JSW, joint space width; MMP, matrix metalloproteinase.
Pairwise forest plots of DMOADs regarding minimum JSW or cartilage volume (a) and withdrawals due to adverse events (b). Random effects model with combined time points was used. ASU, avocado soy unsaponifiables; CI, confidence interval; DMOADs, disease-modifying osteoarthritis drugs; iNOS, inducible nitric oxide synthase; JSW, joint space width; MMP, matrix metalloproteinase; RR, relative risk; SMD, standardised mean of difference.
Head-to-head comparisons for the DMOADs on minimum JSW or cartilage volume and withdrawals due to adverse events. Consistent network meta-analysis model with combined time points was used. Data are SMDs/RRs (95% CI) of the column treatment relative to the row treatment. For minimum JSW or cartilage volume, SMDs higher than 0 favour the column treatment. For withdrawals due to adverse events, RRs lower than 1 favour the column treatment. Significant results are in bold and underscored. ASU, avocado soy unsaponifiables; CI, confidence interval; DMOADs, disease-modifying osteoarthritis drugs; iNOS, inducible nitric oxide synthase; JSW, joint space width; MMP, matrix metalloproteinase; RR, relative risk.
There were significant but clinically low pain-relieving benefits from doxycycline, glucosamine, and vitamin D in the pairwise analysis (Appendix 3.1.1, 3.1.2, and 3.1.3). This was corroborated by network rankings: doxycycline (network SMD − 0.20; CI [− 0.39, − 0.01]; SUCRA 88.7; rank 1), glucosamine (− 0.15; [− 0.25, − 0.05]; 84.7; 2), and vitamin D (− 0.15; [− 0.27, − 0.03]; 83.4; 3) (Appendix 3.1.6, 3.1.7, and 3.1.8). A similar pattern was noted for the outcome measure of joint function, and the pairwise analysis detected favorable results for vitamin D and glucosamine (Appendix 3.2.1, 3.2.2, and 3.2.3). NMA further confirmed the positive functional effects for vitamin D (network SMD −0.18; CI [0.31, −0.06]; SUCRA 87.2; rank 1), glucosamine (−0.17; [−0.28, −0.07]; 84.8; 2), and chondroitin (−0.15; [−0.26, −0.03]; 78.6; 3) (Appendix 3.2.6, 3.2.7, and 3.2.8). Clustered ranking methods demonstrated that glucosamine and chondroitin were more effective than other DMOADs in slowing down structural changes, promoting pain management, and improving joint functions (Appendices 3.1.9, 3.2.9, 3.3).
Based on the NMA results (Appendix 4.3.2 and 4.3.3 and Fig. 4), withdrawals due to adverse events were significantly higher amongst those patients taking MMP inhibitors (network RR 3.80; CI [1.42, 10.14]; SUCRA 2.7; Rank 12), diacerein (2.14; [1.45, 3.18]; 10.9; 11), and doxycycline (1.69; [1.03, 2.75]; 22.1; 10). The clustered ranking with the structural outcome (Fig. 5) revealed high safety for glucosamine, chondroitin, bisphosphonate, strontium, and licofelone. For those agents with significantly negative safety profiles, the most frequent adverse events were musculoskeletal issues for MMP inhibitors and gastrointestinal discomforts for diacerein and doxycycline (Appendices 4.1, 4.2).
Clustered ranking for the DMOADs on minimum JSW or cartilage volume and withdrawals due to adverse events. The plot is based on the clustered analysis of SUCRA values (horizontal and vertical axes values). Treatments lying in the upper right corner are considered to perform well for both outcomes. Each color represents a group of treatments that belong to the same cluster. ASU, avocado soy unsaponifiables; DMOADs, disease-modifying osteoarthritis drugs; iNOS, inducible nitric oxide synthase; JSW, joint space width; MMP, matrix metalloproteinase; SUCRA, surface under the cumulative ranking curves.
Additional subgroup NMAs were undertaken to assess each outcome for knees and hips separately (Appendix 6.1). The structural modifying effect of glucosamine appeared statistically more powerful at the knee than the hip, while diacerein seemed the most effective agent to structural changes at the hip. Bisphosphonate and glucosamine have the best statistical results for relieving pain and improving hip joint function, respectively. In addition, the subgroup analysis was also performed on the glucosamine and chondroitin regarding dosages, dosing schedules, or product subtypes indicated no significant difference in outcomes (Appendix 6.3.1 and 6.3.2). The analysis of publication bias suggested that excluding outliers led to little change in the rankings (Appendix 6.2).
We graded the strength of our conclusions to be of moderate quality for most of the comparisons the outcomes assessed (details in Appendix 1.4 and Appendix 5).
DISCUSSION
In our review, we found moderate-quality evidence that glucosamine and chondroitin had statistically significant but marginally small structural and symptomatic effects, in addition to favorable safety profiles. Statistically, strontium displayed therapeutic benefit in terms of structure modification, while vitamin D displayed improvement in symptoms. Doxycycline also showed promising rankings in terms of structure and function, but its clinical use may be hindered by safety issues. The therapeutic effects of diacerein, bisphosphonate, iNOS inhibitors, ASU, and licofelone on OA were not as significant as the medications indicated above, while MMP inhibitors and vitamin E promoted little structural or symptomatic improvement.
To our knowledge, this study is one of the most comprehensive systematic reviews performed on a wide range of DMOAD candidates for the treatment of hip and knee OA. The predefined criterion for including trials allowed the assessment of the long-term effects. Most of the trials provided direct measurements, ensuring the reliability of the results. Although joint space narrowing from X-rays is a validated primary endpoint in assessing structural progression, we also exploited MRI findings,30, 33, 46, 47 for its high sensitivity. Pieces of information were maximally preserved when combinations of dosage groups and time points were employed to avoid multiple testing. Additional subgroup analyses assessed the relative merit of different time points, dosages, regimens, or product sources. Although knee and hip osteoarthritis may share similar etiology, presentations, and treatment guidelines,57 sensitivity analyses of joint subgroups were performed to explore possible distinctive effects.
It is worth noting that the magnitude of the effect sizes of all the included drugs in our study was probably too small to be clinically meaningful. We conclude that none of our included DMOAD candidates has convincing long-term disease-modifying abilities. However, for most of the included medications, the analyses suggest improved structural modifying effects over time, though these effects were not statistically significant. Future studies should include long-term interventions to assess potential structural modifying effects for DMOADs.
In addition, distinctions between different drugs on the hip and knee joint suggest that OA could display distinct pathophysiological mechanisms and respond differentially to pharmacological interventions at different joint types.59 In our study, it was interesting to see that symptomatic improvement was associated with structural changes for some agents (glucosamine, chondroitin), but not for others (vitamin D, strontium ranelate). While the dissociation between the radiographic stage of OA and the severity of symptoms has long been recognized,60, 61 this remains a subject of debate.62 Some evidence suggests the involvement of different etiological pathways as the causes of structural damage and symptomology.63, 64
Previous meta-analyses have suggested conflicting or ambiguous structural and symptomatic efficacies for glucosamine and chondroitin in OA treatment,5–8 which may largely be attributable to the different inclusion criteria. In our study, with the duration of 1 year and above, statistically significant top rankings were achieved for both medications, although the clinical meaningfulness needs further investigation. Doxycycline, believed to have cartilage protective effects through collagen degradation inhibition,65 was ranked highly by our study in terms of efficacies; however, it showed an unsatisfactory safety profile. Interestingly, a previous meta-analysis,66 which included the short-term application of doxycycline (< 1 year), showed reduced benefits and increased safety when compared to our study. Strontium ranelate presented promising structural modifying effects in our study and is believed to suppress subchondral bone resorption and stimulate cartilage matrix formation.67 However, strontium had no symptomatic relief; this may be explained by the different etiological pathways involved in OA structural damage and symptomology.64 While our safety profile of strontium was acceptable for daily treatment, caution must be exercised as the drug appears to cause serious cardiovascular side effects when treating osteoporosis.68, 69 In contrast, vitamin D, as shown in previous studies5, 70, 71 and this study, showed few structural effects, but demonstrated a potential to improve symptoms. Hence, combining vitamin D with structure-modifying agents such as glucosamine could be theoretically beneficial, though studies need to be done to verify this. It is possible that particular drug regimens could be combined to target different pathways for the optimized treatment of OA. Diacerein, a purified compound with an anthraquinonic structure, was shown to be beneficial for cartilage by inhibiting cytokine production and activation,41, 42 and a recent meta-analysis,72 including some short-term studies (< 1 year), suggested that diacerein had minimal effects on pain reduction and questionable relevance to joint space narrowing. Our study confirmed that for long-term treatment diacerein was not the best agent to choose. Although it provided structural effects at the hip joint, safety issues could limit its use.73 Agents suppressing bone turnover, including bisphosphonates, have been associated with fewer subchondral bone lesions,40 a source of osteoarthritic pain. Despite limited evidence74 that bisphosphonates are effective in the treatment of OA, our study suggests hip pain reduction for alendronate. It would be interesting to see if studies could differentiate the effects of different bisphosphonates on hip and knee joints, and for those with both OA and osteoporosis, bisphosphonates such as alendronate may be a first-line therapy. With regard to the other agents investigated, iNOS inhibitors, ASU, licofelone, MMP inhibitors, and vitamin E, all showed no significant outcomes in either structural progression or symptomatic relief in our study.
Our study has several limitations. First, several DOMADs9 were only recently. For five candidate drugs, we identified only one eligible trial for each,21, 22, 29, 34, 35 thereby making our analyses susceptible to false-negative errors. Secondly, we were unable to perform a stratified analysis of OA stage (Kellgren-Lawrence). Although some of the trials22, 29–32, 35–37, 41, 43, 44, 48, 50, 52, 53 provided information about patients’ OA stages, none of them stratified the outcome data according to the staging. Third, despite the assumption that all trials were jointly randomizable for NMA, the potential barriers to transitivity such as the various durations of follow-up and different usage of medications could make our results questionable. In this study, we combined the dosage and time groups to minimize this potential bias. The combination of dosages may represent a slight underestimation of the desired SD (84), and the combination of time points conservatively (set correlations at 1 comparing to the widely used 0.5) may slightly underestimate the significance. Additional analyses were made with correlations set at 0 for the time points combination, with no obvious change to the effects. Fourth, there were few publications with direct comparisons (14%) between medications, and as a result, the medium heterogeneity and inconsistency presented in our study should be interpreted with caution, given the fact that a star-shaped network limits the possibility to detect both. However, we have provided the confidence intervals for the heterogeneity estimates for the readers to assess. Caution also needs to be employed when interpreting the clustered ranking plots, since they are not based on joint analyses of the two completely independent outcomes. Considering the limited clinical meaningfulness of candidate drugs, clustered ranking plots can be misleading if only taking into account the statistical ranking.
In conclusion, none of the 12 classes of included drugs could be confirmed as clinically effective DMOADs, although some of them showed promising potential in this regard. It is hoped that this review will help physicians, patients, and researchers make informed decisions regarding disease-modifying OA candidate drugs for treatment, clinical studies, and basic research into OA.
References
Woolf A. The Bone and Joint Decade 2000-2010. Ann Rheum Dis. 2000;59(2):81-2.
Litwic A, Edwards M, Dennison E, Cooper C. Epidemiology and Burden of Osteoarthritis. Br Med Bull. 2013;105:185-99.
Desai SP SD, Abramson SB, Buckley L, Crofford LJ, Cush JC, Lovell DJ, Saag KG. Recommendations for use of selective and nonselective nonsteroidal antiinflammatory drugs: an American College of Rheumatology white paper. Arthritis Rheum. 2008;59(8):1058-73.
Hunter DJ. Pharmacologic therapy for osteoarthritis--the era of disease modification. Nat Rev Rheumatol. 2011;7(1):13-22.
Gallagher B, Tjoumakaris FP, Harwood MI, Good RP, Ciccotti MG, Freedman KB. Chondroprotection and the prevention of osteoarthritis progression of the knee: a systematic review of treatment agents. Am J Sports Med. 2015;43(3):734-44.
Richy F, Bruyere O, Ethgen O, Cucherat M, Henrotin Y, Reginster JY. Structural and symptomatic efficacy of glucosamine and chondroitin in knee osteoarthritis: a comprehensive meta-analysis. Arch Intern Med. 2003;163(13):1514-22.
Zeng C, Wei J, Li H, Wang YL, Xie DX, Yang T, et al. Effectiveness and safety of Glucosamine, chondroitin, the two in combination, or celecoxib in the treatment of osteoarthritis of the knee. Sci Rep. 2015;5:16827.
Wandel S, Jüni P, Tendal B, Nüesch E, Villiger PM, Welton NJ, et al. Effects of glucosamine, chondroitin, or placebo in patients with osteoarthritis of hip or knee: network meta-analysis. BMJ. 2010;341:c4675.
Karsdal MA, Michaelis M, Ladel C, Siebuhr AS, Bihlet AR, Andersen JR, et al. Disease-modifying treatments for osteoarthritis (DMOADs) of the knee and hip: lessons learned from failures and opportunities for the future. Osteoarthr Cartil. 2016;24(12):2013-21.
Hunter DJ, Zhang W, Conaghan PG, Hirko K, Menashe L, Reichmann WM, et al. Responsiveness and reliability of MRI in knee osteoarthritis: a meta-analysis of published evidence. Osteoarthr Cartil. 2011;19(5):589-605.
Ganz R, Leunig M, Leunig-Ganz K, Harris WH. The etiology of osteoarthritis of the hip: an integrated mechanical concept. Clin Orthop Relat Res. 2008;466(2):264-72.
Felson DT, Lawrence RC, Dieppe PA, Hirsch R, Helmick CG, Jordan JM, et al. Osteoarthritis: new insights. Part 1: the disease and its risk factors. Ann Intern Med. 2000;133(8):635-46.
Zhang W, Doherty M. EULAR recommendations for knee and hip osteoarthritis: a critique of the methodology. Br J Sports Med. 2006;40(8):664-9.
Barr A, Conaghan P. Disease-modifying osteoarthritis drugs (DMOADs): what are they and what can we expect from them? 2013. 189–96 p
Qvist P, Bay-Jensen A-C, Christiansen C, Dam EB, Pastoureau P, Karsdal MA. The disease modifying osteoarthritis drug (DMOAD): Is it in the horizon? Pharmacol Res. 2008;58(1):1-7.
Higgins JPT GS. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. The Cochrane Collaboration. 2011.
Agency EM. GUIDELINE ON CLINICAL INVESTIGATION OF MEDICINAL PRODUCTS USED IN THE TREATMENT OF OSTEOARTHRITIS. In: USE CFMPFH, editor. 2020.
Administration USFaD. Clinical Development Programs for Drugs, Devices, and Biological Products for the Treatment of Rheumatoid Arthritis (RA) February 1999.
Conaghan PG, Hunter DJ, Maillefert JF, Reichmann WM, Losina E. Summary and recommendations of the OARSI FDA osteoarthritis Assessment of Structural Change Working Group. Osteoarthr Cartil. 2011;19(5):606-10.
McAlindon TE, Nuite M, Krishnan N, Ruthazer R, Price LL, Burstein D, et al. Change in knee osteoarthritis cartilage detected by delayed gadolinium enhanced magnetic resonance imaging following treatment with collagen hydrolysate: a pilot randomized controlled trial. Osteoarthr Cartil. 2011;19(4):399-405.
Raynauld JP, Martel-Pelletier J, Bias P, Laufer S, Haraoui B, Choquette D, et al. Protective effects of licofelone, a 5-lipoxygenase and cyclo-oxygenase inhibitor, versus naproxen on cartilage loss in knee osteoarthritis: a first multicentre clinical trial using quantitative MRI. Ann Rheum Dis. 2009;68(6):938-47.
Reginster JY, Badurski J, Bellamy N, Bensen W, Chapurlat R, Chevalier X, et al. Efficacy and safety of strontium ranelate in the treatment of knee osteoarthritis: results of a double-blind, randomised placebo-controlled trial. Ann Rheum Dis. 2013;72(2):179-86.
Altman R, Brandt K, Hochberg M, Moskowitz R, Bellamy N, Bloch DA, et al. Design and conduct of clinical trials in patients with osteoarthritis: recommendations from a task force of the Osteoarthritis Research Society. Results from a workshop. Osteoarthr Cartil. 1996;4(4):217-43.
Juni P, Reichenbach S, Dieppe P. Osteoarthritis: rational approach to treating the individual. Best Pract Res Clin Rheumatol. 2006;20(4):721-40.
Cohen J. Statistical Power Analysis for the Behavioral Sciences 1988. 567 p.
Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557-60.
Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. Introduction to Meta-Analysis: John Wiley & Sons, Ltd; 2009. i-xxix p.
Chaimani A, Higgins JPT, Mavridis D, Spyridonos P, Salanti G. Graphical Tools for Network Meta-Analysis in STATA. PLoS One. 2013;8(10):e76654.
Hellio le Graverand MP, Clemmer RS, Redifer P, Brunell RM, Hayes CW, Brandt KD, et al. A 2-year randomised, double-blind, placebo-controlled, multicentre study of oral selective iNOS inhibitor, cindunistat (SD-6010), in patients with symptomatic osteoarthritis of the knee. Ann Rheum Dis. 2013;72(2):187-95.
Wluka AE, Stuckey S, Brand C, Cicuttini FM. Supplementary vitamin E does not affect the loss of cartilage volume in knee osteoarthritis: a 2 year double blind randomized placebo controlled study. J Rheumatol. 2002;29(12):2585-91.
Arden NK, Cro S, Sheard S, Dore CJ, Bara A, Tebbs SA, et al. The effect of vitamin D supplementation on knee osteoarthritis, the VIDEO study: a randomised controlled trial. Osteoarthr Cartil. 2016;24(11):1858-66.
McAlindon T, LaValley M, Schneider E, Nuite M, Lee JY, Price LL, et al. Effect of vitamin D supplementation on progression of knee pain and cartilage volume loss in patients with symptomatic osteoarthritis: a randomized controlled trial. JAMA. 2013;309(2):155-62.
Jin X, Jones G, Cicuttini F, Wluka A, Zhu Z, Han W, et al. Effect of Vitamin D Supplementation on Tibial Cartilage Volume and Knee Pain Among Patients With Symptomatic Knee Osteoarthritis: A Randomized Clinical Trial. JAMA. 2016;315(10):1005-13.
Krzeski P, Buckland-Wright C, Balint G, Cline GA, Stoner K, Lyon R, et al. Development of musculoskeletal toxicity without clear benefit after administration of PG-116800, a matrix metalloproteinase inhibitor, to patients with knee osteoarthritis: a randomized, 12-month, double-blind, placebo-controlled study. Arthritis Res Ther. 2007;9(5):R109.
Brandt KD, Mazzuca SA, Katz BP, Lane KA, Buckwalter KA, Yocum DE, et al. Effects of doxycycline on progression of osteoarthritis: results of a randomized, placebo-controlled, double-blind trial. Arthritis Rheum. 2005;52(7):2015-25.
Maheu E, Cadet C, Marty M, Moyse D, Kerloch I, Coste P, et al. Randomised, controlled trial of avocado-soybean unsaponifiable (Piascledine) effect on structure modification in hip osteoarthritis: the ERADIAS study. Ann Rheum Dis. 2014;73(2):376-84.
Lequesne M, Maheu E, Cadet C, Dreiser RL. Structural effect of avocado/soybean unsaponifiables on joint space loss in osteoarthritis of the hip. Arthritis Rheum. 2002;47(1):50-8.
Spector TD, Conaghan PG, Buckland-Wright JC, Garnero P, Cline GA, Beary JF, et al. Effect of risedronate on joint structure and symptoms of knee osteoarthritis: results of the BRISK randomized, controlled trial [ISRCTN01928173]. Arthritis Res Ther. 2005;7(3):R625-33.
Nishii T, Tamura S, Shiomi T, Yoshikawa H, Sugano N. Alendronate treatment for hip osteoarthritis: prospective randomized 2-year trial. Clin Rheumatol. 2013;32(12):1759-66.
Bingham CO, 3rd, Buckland-Wright JC, Garnero P, Cohen SB, Dougados M, Adami S, et al. Risedronate decreases biochemical markers of cartilage degradation but does not decrease symptoms or slow radiographic progression in patients with medial compartment osteoarthritis of the knee: results of the two-year multinational knee osteoarthritis structural arthritis study. Arthritis Rheum. 2006;54(11):3494-507.
Pham T, Le Henanff A, Ravaud P, Dieppe P, Paolozzi L, Dougados M. Evaluation of the symptomatic and structural efficacy of a new hyaluronic acid compound, NRD101, in comparison with diacerein and placebo in a 1 year randomised controlled study in symptomatic knee osteoarthritis. Ann Rheum Dis. 2004;63(12):1611-7.
Dougados M, Nguyen M, Berdah L, Mazieres B, Vignon E, Lequesne M. Evaluation of the structure-modifying effects of diacerein in hip osteoarthritis: ECHODIAH, a three-year, placebo-controlled trial. Evaluation of the Chondromodulating Effect of Diacerein in OA of the Hip. Arthritis Rheum. 2001;44(11):2539-47.
Uebelhart D, Malaise M, Marcolongo R, de Vathaire F, Piperno M, Mailleux E, et al. Intermittent treatment of knee osteoarthritis with oral chondroitin sulfate: a one-year, randomized, double-blind, multicenter study versus placebo. Osteoarthr Cartil. 2004;12(4):269-76.
Kahan A, Uebelhart D, De Vathaire F, Delmas PD, Reginster JY. Long-term effects of chondroitins 4 and 6 sulfate on knee osteoarthritis: the study on osteoarthritis progression prevention, a two-year, randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2009;60(2):524-33.
Michel BA, Stucki G, Frey D, De Vathaire F, Vignon E, Bruehlmann P, et al. Chondroitins 4 and 6 sulfate in osteoarthritis of the knee: a randomized, controlled trial. Arthritis Rheum. 2005;52(3):779-86.
Wildi LM, Raynauld JP, Martel-Pelletier J, Beaulieu A, Bessette L, Morin F, et al. Chondroitin sulphate reduces both cartilage volume loss and bone marrow lesions in knee osteoarthritis patients starting as early as 6 months after initiation of therapy: a randomised, double-blind, placebo-controlled pilot study using MRI. Ann Rheum Dis. 2011;70(6):982-9.
Railhac JJ, Zaim M, Saurel AS, Vial J, Fournie B. Effect of 12 months treatment with chondroitin sulfate on cartilage volume in knee osteoarthritis patients: a randomized, double-blind, placebo-controlled pilot study using MRI. Clin Rheumatol. 2012;31(9):1347-57.
Uebelhart D, Thonar EJ, Delmas PD, Chantraine A, Vignon E. Effects of oral chondroitin sulfate on the progression of knee osteoarthritis: a pilot study. Osteoarthr Cartil.. 1998;6 Suppl A:39-46.
Sawitzke AD, Shi H, Finco MF, Dunlop DD, Harris CL, Singer NG, et al. Clinical efficacy and safety of glucosamine, chondroitin sulphate, their combination, celecoxib or placebo taken to treat osteoarthritis of the knee: 2-year results from GAIT. Ann Rheum Dis. 2010;69(8):1459-64.
Sawitzke AD, Shi H, Finco MF, Dunlop DD, Bingham CO, 3rd, Harris CL, et al. The effect of glucosamine and/or chondroitin sulfate on the progression of knee osteoarthritis: a report from the glucosamine/chondroitin arthritis intervention trial. Arthritis Rheum. 2008;58(10):3183-91.
Rozendaal RM, Koes BW, van Osch GJ, Uitterlinden EJ, Garling EH, Willemsen SP, et al. Effect of glucosamine sulfate on hip osteoarthritis: a randomized trial. Ann Intern Med. 2008;148(4):268-77.
Reginster JY, Deroisy R, Rovati LC, Lee RL, Lejeune E, Bruyere O, et al. Long-term effects of glucosamine sulphate on osteoarthritis progression: a randomised, placebo-controlled clinical trial. Lancet. 2001;357(9252):251-6.
Pavelka K, Gatterova J, Olejarova M, Machacek S, Giacovelli G, Rovati LC. Glucosamine sulfate use and delay of progression of knee osteoarthritis: a 3-year, randomized, placebo-controlled, double-blind study. Arch Intern Med. 2002;162(18):2113-23.
Kawasaki T, Kurosawa H, Ikeda H, Kim SG, Osawa A, Takazawa Y, et al. Additive effects of glucosamine or risedronate for the treatment of osteoarthritis of the knee combined with home exercise: a prospective randomized 18-month trial. J Bone Miner Metab. 2008;26(3):279-87.
Fransen M, Agaliotis M, Nairn L, Votrubec M, Bridgett L, Su S, et al. Glucosamine and chondroitin for knee osteoarthritis: a double-blind randomised placebo-controlled clinical trial evaluating single and combination regimens. Ann Rheum Dis. 2015;74(5):851-8.
Bruyere O, Pavelka K, Rovati LC, Gatterova J, Giacovelli G, Olejarova M, et al. Total joint replacement after glucosamine sulphate treatment in knee osteoarthritis: results of a mean 8-year observation of patients from two previous 3-year, randomised, placebo-controlled trials. Osteoarthr Cartil. 2008;16(2):254-60.
Practitioners TRACoG. Guideline for the management of knee and hip osteoarthritis 2nd Edition. East Melbourne, Vic: RACGP. 2018.
Ryan R HS. How to GRADE the quality of the evidence. Cochrane Consumers and Communication Group. 2016.
Zhang W, Doherty M. EULAR recommendations for knee and hip osteoarthritis: a critique of the methodology. Br J Sports Med. 2006;40(8):664-9.
Hannan MT, Felson DT, Pincus T. Analysis of the discordance between radiographic changes and knee pain in osteoarthritis of the knee. J Rheumatol. 2000;27(6):1513-7.
Bedson J, Croft PR. The discordance between clinical and radiographic knee osteoarthritis: a systematic search and summary of the literature. BMC Musculoskelet Disord. 2008;9:116.
Duncan R, Peat G, Thomas E, Hay E, McCall I, Croft P. Symptoms and radiographic osteoarthritis: not as discordant as they are made out to be? Ann Rheum Dis. 2007;66(1):86-91.
Torres L, Dunlop DD, Peterfy C, Guermazi A, Prasad P, Hayes KW, et al. The relationship between specific tissue lesions and pain severity in persons with knee osteoarthritis. Osteoarthr Cartil. 2006;14(10):1033-40.
Felson DT. Clinical practice. Osteoarthritis of the knee. N Engl J Med. 2006;354(8):841-8.
Amin AR, Attur MG, Thakker GD, Patel PD, Vyas PR, Patel RN, et al. A novel mechanism of action of tetracyclines: effects on nitric oxide synthases. Proc Natl Acad Sci U S A. 1996;93(24):14014-9.
da Costa BR, Nuesch E, Reichenbach S, Juni P, Rutjes AW. Doxycycline for osteoarthritis of the knee or hip. Cochrane Database Syst Rev. 2012;11:CD007323.
Yu D-g, Ding H-f, Mao Y-q, Liu M, Yu B, Zhao X, et al. Strontium ranelate reduces cartilage degeneration and subchondral bone remodeling in rat osteoarthritis model. Acta Pharmacol Sin. 2013;34(3):393-402.
Reginster J-Y. Cardiac concerns associated with strontium ranelate. Expert Opin Drug Saf. 2014;13(9):1209-13.
Vestergaard P. New strategies for osteoporosis patients previously managed with strontium ranelate. Ther Adv Musculoskelet Dis. 2014;6(6):217-25.
Diao N, Yang B, Yu F. Effect of vitamin D supplementation on knee osteoarthritis: A systematic review and meta-analysis of randomized clinical trials. Clin Biochem. 2017;50(18):1312-6.
Gao XR, Chen YS, Deng W. The effect of vitamin D supplementation on knee osteoarthritis: A meta-analysis of randomized controlled trials. Int J Surg. 2017;46:14-20.
Fidelix TS, Macedo CR, Maxwell LJ, Fernandes Moca Trevisani V Diacerein for osteoarthritis. Cochrane Database Syst Rev. 2014(2):CD005117.
Panova E, Jones G. Benefit-risk assessment of diacerein in the treatment of osteoarthritis. Drug Saf. 2015;38(3):245-52.
Davis AJ, Smith TO, Hing CB, Sofat N. Are bisphosphonates effective in the treatment of osteoarthritis pain? A meta-analysis and systematic review. PLoS One. 2013;8(9):e72714.
Author information
Authors and Affiliations
Contributions
Wei Yang (WY), Qi Zhuo (QZ), Cheng Sun (CS), Sheng Qin He (SQH), Ji Ying Chen (JYC), and Yan Wang (YW) conceived and designed the study. WY, CS, and QZ selected the articles and extracted the data. WY, QZ, CS, and SQH analyzed the data. WY, QZ, CS, SQH, JYC, and YW interpreted the data and contributed to the writing of the manuscript. WY, CS, SQH, YW, JYC, and QZ contributed equally to this work.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they do not have a conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yang, W., Sun, C., He, S.Q. et al. The Efficacy and Safety of Disease-Modifying Osteoarthritis Drugs for Knee and Hip Osteoarthritis—a Systematic Review and Network Meta-Analysis. J GEN INTERN MED 36, 2085–2093 (2021). https://doi.org/10.1007/s11606-021-06755-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11606-021-06755-z