Abstract
Background
Lean management has been successfully employed in healthcare to improve outcomes and efficiencies. Facilitation is increasingly being used to support evidence-based practice uptake in healthcare. However, while both Lean and Facilitation are used in healthcare quality improvement, limited research has explored their integration and the sustainability of their combined effects.
Objective
To improve hepatitis C virus (HCV) screening rates among persons born between 1945 and 1965 through the design and evaluation of a multi-modal Lean-Facilitation intervention (LFI) for Department of Veterans Affairs primary care community clinics.
Design
We conducted a mixed methods quasi-experimental evaluation in eight clinics, guided by the integrated Promoting Action on Research Implementation in Health Services framework.
Participants
We engaged regional and local leadership (N = 9), implemented our LFI with clinicians and staff (N = 68), and conducted summative interviews with participants (N = 13).
Intervention
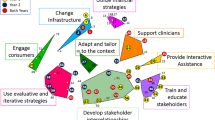
The LFI included six implementation strategies: (1) external facilitation, (2) stakeholder engagement, (3) champion activation, (4) rapid process improvement sessions, (5) Plan-Do-Study-Act cycles, and (6) audit-feedback.
Measures
The primary outcome was rate of new HCV screening among previously untested patients with a primary care visit. Using interrupted time series, we analyzed intervention and time effects on HCV testing rates, and administered organizational readiness surveys, conducted summative qualitative interviews, and tracked facilitation events.
Results
The LFI was associated with significant, immediate, and sustained increases in HCV testing. No change was detected at matched comparison clinics. Staff accepted the LFI and the philosophy of “bottom-up” solution development yet had mixed feedback on its appropriateness and feasibility. Enablers of implementation and early sustainment included lower satisfaction with baseline HCV testing processes and staff culture, while later sustainment was related to implementation climate support, measurement, and evaluation.
Conclusions
High-reach and relatively low effort, but persistent intervention led to significant improvement in guideline-concordant HCV testing rates which were sustained.
Trial Registration
ClinicalTrials.gov Identifier: NCT02936648
Similar content being viewed by others
BACKGROUND
Recent advances in implementation frameworks and models, definitions of discrete strategies, and early efforts to specify mechanisms of change are charging the sciences of implementation and quality improvement to align in addressing the challenges of translating research to practice.1,2,3,4
Lean management (Lean), or the “philosophy of optimization” popularized by the Toyota Production System, has been employed successfully in healthcare quality improvement (QI).5,6,7 Lean originates in management and industrial engineering, and is grounded in five principles: understand value from the customer perspective, streamline workflows, maximize productivity, reduce waste, and evaluate and iterate.8,9,10 Lean management begins with transforming work culture, then redesigning and continually refining processes.
In tandem with the growing application of Lean in healthcare, the use of implementation facilitation to support practice change has been advancing.11 Facilitation typically combines implementation strategies into a multi-faceted intervention to build the absorptive capacity of a team, while attending to the inner and outer context in which the team operates.12, 13
A hallmark of both Lean and implementation facilitation is emphasis on experiential collaborative learning and consensus-based problem-solving. While Lean and Facilitation are now commonly used in healthcare QI, the interaction and integration of the two, and sustainability of their effects have rarely been studied using quasi-experimental evaluation methods.14 Therefore, this article provides a novel conceptualization and empirical investigation of how the two operate together.
As the largest integrated healthcare system in the USA, the Department of Veterans Affairs (VA) is well suited to study strategies for evidence-based practice guideline implementation in primary care. We selected hepatitis C virus (HCV) for improvement because: (1) HCV is the most common bloodborne infection in the USA, and affects veterans at three times the rate of the general population; (2) all major guideline-setting bodies in the USA recommend those born between 1945 and 1965 (Baby Boomer birth cohort) be tested for HCV, regardless of behavioral risk factors; and (3) newer medications make HCV curable, preventing morbidity and mortality, and reducing healthcare system costs.15,16,17,18 Still, as recently as 2015, 31% of veterans in VA care had never been tested for HCV.19
Guidelines alone are seldom sufficient in initiating and maintaining practice change.20 Design of this intervention was informed by our formative work on primary care provider perceptions towards updated HCV screening guidelines, which identified patient-, provider-, and system-level factors promoting or inhibiting implementation, including patient reluctance to accept testing, diverse provider opinions on the value of expanded testing, and inefficient clinical reminder tools and lab ordering protocols.21 We anticipated that specific clinic experiences compounded barriers, and thus would require a multi-component Lean-Facilitation intervention (LFI) addressing intrapersonal (i.e., awareness, knowledge, motivation), interpersonal (i.e., enhancing communication skills), organizational (i.e., culture and climate), and structural (i.e., increasing access to phlebotomy) factors. We selected facilitation to drive the intervention and simultaneously incorporate both transformational (i.e., champion development) and transactional (i.e., audit and feedback) strategies, while attending to a dynamically changing context.22
The purpose of this work was to (1) design an LFI for HCV birth cohort testing improvement in VA community clinics, (2) evaluate its implementation and sustainability, and (3) evaluate clinical impact.
METHODS
Study Design and Setting
This mixed methods interrupted time series quasi-experimental quality improvement study was conducted in eight VA New England community clinics—four QI clinics and four matched comparison clinics. The four QI clinics were selected in collaboration with regional leadership and represent diversity with respect to practice size, patient volume, geography, and HCV testing performance (Table 1).
Data were collected over four periods: pre-implementation (October 2015 to March 2016), implementation (April 2016 to August 2016), early sustainment (September 2016 to August 2017), and later sustainment (September 2017 to June 2018). This project was conducted in accordance with VHA Handbook (1058.05) quality improvement guidelines and was deemed exempt from review by the VA Bedford VA Institutional Review Board.23
Lean-Facilitation Intervention
Lean-Facilitation intervention (LFI) development was informed by a formative evaluation, and literature review of Lean methods and implementation facilitation. This resulted in a multi-faceted 5-month intervention composed of six implementation strategies: (1) facilitation delivered by a multi-disciplinary QI team including infectious disease clinicians (AG, DT), a process improvement specialist (AP), industrial engineers (KD, WL), and a public health professional (VY); (2) stakeholder engagement; (3) champion activation; (4) clinic-wide on-site rapid process improvement sessions; (5) Plan-Do-Study-Act (PDSA) small tests of change; and (6) audit and feedback. Appendix 1 details the strategies implemented guided by Proctor’s specifications (actor, the action, action targets, temporality, dose, implementation outcomes addressed, and theoretical justification).24, 25 Comparison clinics received no intervention from the QI team.
Data Collection and Outcomes
Measure selection was guided by the integrated Promoting Action on Research Implementation in Health Services (i-PARIHS) framework to help determine factors related to implementation success. Drawing upon behavioral, learning, innovation, and implementation theories, the i-PARIHS framework posits that successful implementation is a function of the interdependent relationships of the innovation’s characteristics, recipient factors, and the inner and outer context as propelled by the active ingredient of facilitation.26 Both quantitative (organizational readiness surveys, facilitation tracking tools, administrative data) and qualitative (field notes and qualitative interviews) data were collected and aligned to i-PARIHS domains of innovation, recipients, context, and facilitation, to evaluate implementation and effectiveness.
Effectiveness
To determine the clinical effectiveness of the LFI, the primary outcome was the rate of new HCV screening, defined as the proportion of previously untested patients with primary care visit and newly tested within 30 days. Patients eligible and not tested were classified as “missed testing opportunities.” The two secondary outcomes were (1) proportion of untested with documentation of testing refusal within a nationally standardized clinical reminder alert and (2) HCV seropositivity, or “positive yield,” defined as the proportion of positive antibody results among those newly tested each month. All data were extracted from VA’s Corporate Data Warehouse obtained through diagnosis codes (ICD-9-CM and ICD-10-CM), laboratory data (Logical Observation Identifiers Names and Codes, LOINC), procedure codes (CPT4), and health factors. We obtained clinic characteristics including data on primary care patient volume and provider panel sizes from the VA Patient-Aligned Care Teams Compass Cube.
Implementation
We tracked LFI implementation and facilitation activities including date, length of time, and staff involved on a standardized facilitation form completed by the external facilitator (VY).27 We also collected field notes during on-site sessions, facilitator-champion meetings and study team meetings, and gathered email correspondence.
Staff Surveys
To assess i-PARIHS innovation, recipient, and context domains, we administered an organizational survey using Jacobs’ Implementation Climate measures and an abbreviated Organizational Readiness to Change Assessment (ORCA).28, 29 The 35-item 5-point Likert scale survey was administered to providers and staff at two time points: (1) immediately before the first on-site QI session and (2) again at the end of the 5-month implementation period. The survey asked about perceptions of the evidence of HCV testing, staff and leadership culture, leadership behavior, measurement and evaluation, implementation climate related to expectations, support, and rewards, and demographics (age, sex, degree, tenure).30, 31
Staff Interviews
At the end of the implementation period, we conducted semi-structured summative qualitative interviews with each clinic champion and other staff. An evaluator (MLD) independent from the facilitation team asked about feasibility, acceptability, impact, and sustainability of the intervention. We used snowball sampling to identify up to four additional staff per clinic to participate in interviews.32 Interviews lasted up to 60 min and detailed notes were taken to produce both verbatim and paraphrased material.
Analysis
We evaluated the effectiveness of the LFI through interrupted time series analysis comparing HCV testing before and after the implementation period and between QI and matched comparison clinics.33 We evaluated within-clinic LFI sustainment by examining differences between the implementation period and early sustainment, and between early sustainment and later sustainment. We also used t tests and Kendall’s tau to examine whether HCV testing refusal rates and HCV positive yield differed across time and between QI and comparison clinics. Sensitivity analyses examined the effects of testing timing, by comparing shorter or longer windows (15-day and 45-day) between primary care visit and lab test.
Other quantitative data from the organizational surveys and facilitation tracking were analyzed using descriptive and bivariate statistics (t test, Wilcox, and Kruskal-Wallis). Changes in organizational survey scores between clinics at baseline and follow-up were examined, as were within-in clinic changes over time. Associations between organizational survey results and HCV testing performance during the four implementation periods (pre-implementation, implementation, early sustainment, and later sustainment) were examined using Spearman’s correlation. All statistical analyses were performed with RStudio v.1.0.153 and p values < .05 were considered statistically significant.
Qualitative data (field notes, email correspondence, and interview notes) were analyzed using rapid deductive content techniques.34,35,36 This analysis identified cross-clinic patterns and differences related to i-PARIHS domain enabling, neutral, or hindering effects. As a final step, qualitative and quantitative data were triangulated.37
RESULTS
First, we present the clinical results, followed by details on LFI implementation and experiences with implementation based on interview, observation, and survey data.
Effectiveness and Sustainment
There were 13,184 VA patients who were born between 1945 and 1965 and attended a primary care outpatient visit over the 5-month intervention period. Of these, 56% had been tested previously and, among the remaining 5829 previously untested patients, 1057 were newly tested. Among those eligible and not tested were 4772 patients considered “missed testing opportunities.”
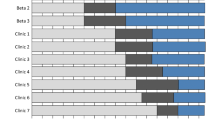
HCV testing of eligible patients increased by 200% at QI clinics during the 5-month implementation period. The proportion of monthly new testing among previously untested patients was more than three times higher at QI clinics as compared with comparison clinics (24.6% vs. 7.9%, p < .001). The monthly HCV testing rate peaked at 32.1% in month 3 of implementation, then declined to 16.1% at the end of the first sustainability year, and to 10.6% in year 2, but remained above the 8.8% baseline rate throughout the sustainability period.
Interrupted time series analysis demonstrated an overall significant intervention effect (p < .001) that was maintained into early sustainment (1 year post LFI), which was over and above the existing trend before the introduction of the LFI (p = 0.54). All four QI clinics compared with matched comparison clinics had higher testing during implementation than during pre-implementation, but improvement and sustainment were not uniform: Clinic A had modest improvement during the LFI (p = .002) then steadily declined to near baseline levels by the end of the sustainment period, clinic B (p < .001) markedly increased testing and maintained elevated testing into early and later sustainment, clinic C did not have statistically significant improvement (p = .45), and clinic D had rapid improvement (p = .01) that was maintained through early sustainment. Sensitivity analyses, adjusting the test acquisition window (days since the primary care visit), did not change findings. Figure 1 displays the four clinic pairs and four implementation periods including a gray box denoting the implementation period.
HCV testing refusal rates documented via the clinical reminder did not vary significantly by intervention arm or implementation period; however, there was an overall downward trend in refusals at QI and comparison clinics (τ = − .59, p < .001). Among those newly tested, HCV antibody positive rates did not differ by intervention arm (5.6% QI vs. 6.0% comparison, p = .31) or during the implementation period at QI clinics (6.3% to 5.6%, p = 0.56) or comparison clinics (7.2% to 4.8%, p = 0.54).
Lean-Facilitation Intervention Implementation
During the pre-implementation period, there were 18 facilitation stakeholder (regional primary care service line director, healthcare system primary care service line managers, and clinic providers) outreach events (emails and telephone calls) to obtain buy-in and develop regional-, clinic-, and champion-level rapport prior to on-site QI sessions at the four clinics. Of the four healthcare system leaders asked to participate, all agreed and nominated local staff to serve as clinic champions. Clinic champions differed by staff type (2 RN, 1 NP, 1 DO), VA tenure, knowledge and attitudes around testing, and communication preferences with the facilitator.
The four QI clinics that participated represented four VA healthcare systems of variable size, geography, and baseline HCV testing performance. QI clinics had three to seven primary care teams composed of a provider (MD/DO/PA/NP), nurse, and medical support assistant/health technician; a total of 68 staff participated. Staff demographics were balanced between the four QI clinics and included nurses (51%), MD/DO/PA/NP (31%), and health technicians (13%), females (60%), ages 40–64 (67%), and with over 11 years of VA experience (36%).
During the 5-month implementation period, there were eight on-site QI sessions, bi-weekly facilitator check-ins with each clinic, ad hoc champion-initiated outreach to the facilitator, and monthly audit and feedback reports. Clinic champions had an average of 33 (range 21–41) interactions with the facilitator, concentrated around the first month of implementation. Facilitation dose/intensity with clinics was largely mediated by champions’ level of engagement and communication style. Two champions (clinics A and C) had sporadic contact with the facilitator and two (clinics B and D) had more consistent contact. During the sustainment period, there was no pre-specified contact between the facilitation team and QI clinics and interaction was negligible.
Organizational Survey
QI clinics differed in several areas (Table 2), including in degree of baseline satisfaction with their HCV testing processes: clinics A, B, C, and D were 40%, 15%, 67%, and 64% satisfied (p = .04), respectively. Clinics with lower satisfaction had greater improvement in testing rates during the implementation period (r = − .30, p = .04). By follow-up, clinics B and D had significantly improved their satisfaction to 83% and 90%, respectively.
While there were no differences between sites on the perception of evidence, and most respondents (73%) agreed HCV birth cohort testing is “supported by scientific evidence,” less than half (42%) agreed that testing is “consistent with clinical practices that have been accepted by VA patients.” There were mixed feelings within clinics about the utility of HCV testing as evidenced by > 1 standard deviation on evidence items (data not shown). When examining perceptions of evidence changes from baseline to follow-up for each clinic separately, clinics A, B, and D had significant improvements. There was a significant inverse relationship between baseline perception of the evidence on testing effectiveness and pre-implementation HCV testing rates (r = − .36, p = .03).
There were baseline differences between clinics in three of the four context subscales. Clinics C and D had relatively consistent and higher scores, while clinic A had lower scores and more diversity in perspectives, and clinic B had lower scores, but less diversity in perspectives based on score standard deviation. When assessing differences between baseline and follow-up scores, only clinic B had significant improvement on implementation climate expectations and rewards items. At baseline, three scales were positively and significantly associated with testing sustainment: implementation climate support (r = .31, p = .04), ORCA staff culture (r = .33, p = .03), ORCA leadership behavior (r = .41, p = .009), and ORCA measurement and evaluation (r = .36, p = .02).
Staff Experiences with the LFI
Thirteen semi-structured qualitative interviews were conducted at the end of the implementation period with clinic champions, providers, and staff. The interviewees were nine women and four men—eight nurses, three physicians, one nurse practitioner, and one health technician; five held managerial roles, one was Lean Yellow Belt certified; and VA experience ranged from 6 months to 34 years.
Overall, staff accepted the LFI and found it appropriate and timely yet did have mixed feedback on the individual implementation strategies within the LFI. Regarding external facilitation, staff unanimously appreciated the localized focus and support from clinical and implementation experts. All leadership approached to participate agreed, but then had limited involvement throughout implementation, except for clinic A which vetted local activities with leadership within a more hierarchical management structure. Still, some staff reported feeling powerless to initiate change, despite knowledge of leadership agreement to participate in the LFI. One staff member expressed hesitancy about “going out on a ledge,” citing locally initiated QI efforts were often not well received by regional leadership. Some participants were generally resistant to QI, as their personal motivations to participate were colored by past QI experiences that were perceived as failures. Nevertheless, staff reported high expectations to conduct birth cohort testing given the recent addition of HCV as a performance measure for regional leaders.
During on-site sessions, most felt that appropriate clinical and QI information was given with a few individual exceptions, including some resistance from physicians who believed they did not need the educational component or who, in some cases, did not agree with the screening guidelines. However, counteracting some apprehension was the motivating factor of recent changes in HCV treatment effectiveness. Clinic B began using direct-acting HCV treatments on-site during the implementation period. In contrast, clinic C experienced technology and construction barriers which reduced clinic visits to higher-need patients which may have diverted providers’ focus away from prevention-oriented care. Also, during implementation, clinic D lost a primary care provider to retirement.
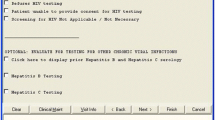
There were diverse opinions about the solution-generation process within and between clinics. Some respondents appreciated self-generated ideas, while others sought more guided direction from the facilitation team and conveyed a “don’t ask us what to do” mentality. For some, there were concerns that the QI team was not well-versed in the local context or implementation issues unique to their clinic. On the other hand, the “bottom-up” Lean approach left respondents “enlightened” and “revved up” because it was unlike other initiatives in which “usually someone changes something and then you stumble upon it.” Figure 2 illustrates an application of Lean process mapping, barrier identification, and multi-voting for patient- and provider-, and system-oriented solutions.
Generally, participants expected the changes their clinics instituted would be sustained either because the changes were small, or—in the place where they were considered substantive and a lot of work—had been incorporated into the workflow of the lowest level staff. Some of the tested changes were permanent solutions, while others more of a stopgap, as one respondent emphasized: “I am not sure in the long term this is the answer but for now it is what we are doing.” A major barrier to sustainment was perceptions of low treatment access, with the strong belief that if treatment access became a barrier then testing would decline. And yet, staff reported being encouraged by more frequent patient-initiated testing requests and patients’ greater agreement to test.
Monthly audit and feedback performance reports were shared with clinic champions for further distribution in the clinic and leadership. The dissemination of performance data was neither consistent nor broad within clinics, and there were mixed statements about the utility of the reports. Some found the monthly reports “shockingly helpful” for monitoring progress and useful for management and decision-making, while others were broadly distrustful of the data or exhibited data denial.
DISCUSSION
The purpose of this study was to design, implement, and evaluate a multi-modal Lean-Facilitation quality improvement intervention to increase HCV testing in primary care community clinics. Overall, the highly successful intervention resulted in a 200% increase in guideline-concordant HCV testing rates. Five months of high-reach and relatively low effort, but persistent facilitation led to improvement in testing which were sustained for 2 years after LFI initiation. Different contextual features were associated with improvement across implementation and sustainment periods. Despite numerous competing priorities and a rapidly changing HCV care landscape, QI clinics accepted the LFI overall yet had variable responses to its subcomponent strategies.
We selected Lean approaches because they are well-suited for lower complexity processes and settings, such as age-based HCV screening and community clinics.38 In contrast, Facilitation is largely agnostic to process and setting complexity and serves as a versatile intervention delivery method. Both Lean and Facilitation operate on the notion that frontline staff are primary problem-solvers who must be activated and supported to ensure successful implementation of evidence into practice.12 Facilitation is grounded in building effective interpersonal relationships, and parallels Lean principles of iterative “pulling” of ideas and active evaluation. It appears that frontline staff can quickly develop familiarity in Lean philosophy and tools, which then become a transferable skillset with application towards other clinical areas. Studying such “cross-pollination” in less linear and more iterative and complex processes is now necessary.
Our findings support the literature that facilitator roles are dynamic and shift with time, and that brief intervention may be sufficient to shift perspectives and practices to both activate and sustain change.39, 40 Although the LFI had five active implementation months, the most labor-intensive segment was the first month of champion activation and on-site sessions. Recent work by Miech et al. has provided clarity on the use of champions in implementation studies, but much remains to be learned about champions’ roles, functions, and integration with other implementation strategies.41 Relatedly, external facilitator withdrawal, including how and when to do so, requires further study.42
We encountered largely positive feedback on the LFI, with several notable areas of improvement. Although a hallmark of Lean is autonomy in solution design, we found staff often sought more substantive involvement and directives from the QI team. This, in part, contradicts the intent of Lean and Facilitation which are based on bottom-up solution generation and may be predicated on psychological safety.43 Also, despite close attention to audit and feedback design elements, staff sometimes displayed difficulty with understanding the reports and effectively using them.44 Other variations of feedback reports may be tested in the future, including more targeted accountability via individualized provider reports, rather than clinic-level reports, and daily or weekly feedback rather than monthly. Although the relative contribution of our intervention components is unknown, their integration was likely synergistic and not simply additive.
Consistent with the literature, our findings also point to the common phenomenon of “program drift,” where the pace of improvement declines over time.45 This is not an unexpected finding given the birth cohort population is finite and testing saturation will be reached; however, understanding the durability of an intervention can help with the design and timing of intervention boosters and other reinforcing strategies. For example, continued reinforcement (be it due to staff turnover, inertia, etc.) with personalized feedback both was requested by participants and is likely necessary for sustainability. Understanding sustainability is an underdeveloped field and requires theoretical development and further empirical study, particularly for finite and time-limited improvement efforts.46, 47
Intervention clinics outperformed comparison clinics during the intervention period; however, comparison clinics did have improvements in testing, albeit delayed and smaller improvement compared with their matched QI clinics. Importantly, HCV seropositivity rates were virtually unchanged at QI clinics despite the threefold testing increase, suggesting the added, newly screened patients were at equally high HCV risk as those previously tested. Likewise, refusals to accept tests did not change significantly. Closer examination of reasons for test refusal are likely necessary, including consideration that recorded “patient refusal” might instead be provider reluctance to offer testing.48
Finally, while shifting away from risk behavior–based to population-based HCV testing was the focus of this work, the emergence of the opioid epidemic further compels clinicians to be more than ever attuned to high risks in this younger cohort of people who inject drugs. The estimated actual new cases of HCV increased nationally by 142% between 2010 and 2016, most notably in the 20–39 age group, and predominantly due to injection drug use exposure.49, 50 As such, in March 2020, the US Preventive Services Task Force expanded its HCV screening recommendation to all adults between ages 18 and 79.51 Whereas one-time testing by age cohort is relatively simple and unambiguous, determining repeat screening intervals for those with continued risk behavior is more complex and continues to be studied. Questions of implementation and de-implementation may be asked here as HCV screening guidelines shift towards universal screening.52
LIMITATIONS
This work has several limitations. Due to a small sample size and quickly changing VA and HCV environments, there are limitations in cross-clinic comparisons and other concerns of confounding. Furthermore, only one intervention clinic had screened < 50% of its population, suggesting a possible ceiling effect. These results may not be generalizable to non-VA settings because there are significant differences between the VA and US population, and community primary care practices may not have similar tools available to implement change.
CONCLUSIONS
HCV screening rates in primary care community clinics tripled and remained elevated above baseline throughout the 2-year sustainability period following our multi-modal Lean-Facilitation QI intervention. By merging Lean process improvement and facilitation implementation, we leveraged their complimentary philosophies and tools, to simultaneously build primary care team culture and skills to successfully improve and sustain HCV testing. However, improvement, management, and implementation science have co-evolved with limited intersection theoretically and empirically. Finally, our findings can be viewed as a response to ongoing calls for implementation scientists to engage with the improvement and management sciences to generate new knowledge.
References
Nilsen P. Making sense of implementation theories, models and frameworks. Implement Sci 2015; 10:53.
Waltz TJ, Powell BJ, Matthieu MM, Damschroder LJ, Chinman MJ, Smith JL, et al. Use of concept mapping to characterize relationships among implementation strategies and assess their feasibility and importance: results from the Expert Recommendations for Implementing Change (ERIC) study. Implement Sci 2015; 10:109.
Powell BJ, Waltz TJ, Chinman MJ, Damschroder LJ, Smith JL, Matthieu MM, et al. A refined compilation of implementation strategies: results from the Expert Recommendations for Implementing Change (ERIC) project. Implement Sci 2015; 10:21.
Lewis CC, Klasnja P, Powell BJ, Lyon AR, Tuzzio L, Jones S, et al. From Classification to Causality: Advancing Understanding of Mechanisms of Change in Implementation Science. Front Public Health 2018; 6.
Kim CS, Spahlinger DA, Kin JM, Billi JE. Lean health care: what can hospitals learn from a world-class automaker? J Hosp Med 2006; 1:191-9.
de Koning H, Verver JPS, van den Heuvel J, Bisgaard S, Does RJMM. Lean Six Sigma in Healthcare. J Healthcare Qual 2006; 28:4-11.
Nelson-Peterson DL, Leppa CJ. Creating an environment for caring using lean principles of the Virginia Mason Production System. J Nurs Admin 2007; 37:287-94.
Kruskal JB, Reedy A, Pascal L, Rosen MP, Boiselle PM. Quality initiatives: lean approach to improving performance and efficiency in a radiology department. Radiographics. 2012; 32:573-87.
Teich ST, Faddoul FF. Lean management-the journey from toyota to healthcare. Rambam Maimonides Med J. 2013; 4:e0007-e.
Woodhouse LD, Toal R, Nguyen T, Keene D, Gunn L, Kellum A, et al. A merged model of quality improvement and evaluation: maximizing return on investment. Health Promot Pract 2013; 14:885-92.
Kirchner JE, Smith JL, Powell BJ, Waltz TJ, Proctor EK. Getting a Clinical Innovation into Practice: An Introduction to Implementation Strategies. Psychiatry Res. 2019.
Berta W, Cranley L, Dearing JW, Dogherty EJ, Squires JE, Estabrooks CA. Why (we think) facilitation works: insights from organizational learning theory. Implement Sci 2015; 10:141.
Baskerville NB, Liddy C, Hogg W. Systematic Review and Meta-Analysis of Practice Facilitation Within Primary Care Settings. Ann Family Med 2012; 10:63-74.
Alagoz E, Chih M-Y, Hitchcock M, Brown R, Quanbeck A. The use of external change agents to promote quality improvement and organizational change in healthcare organizations: a systematic review. BMC Health Serv Res 2018; 18:42.
Dominitz JA, Boyko EJ, Koepsell TD, Heagerty PJ, Maynard C, Sporleder JL, et al. Elevated prevalence of hepatitis C infection in users of United States veterans medical centers. Hepatology. 2005; 41:88-96.
Moyer VA. Screening for hepatitis C virus infection in adults: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 2013; 159:349-57.
Coretti S, Romano F, Orlando V, Codella P, Prete S, Di Brino E, et al. Economic evaluation of screening programs for hepatitis C virus infection: evidence from literature. Risk Manag Healthcare Pol 2015; 8:45-54.
Rein DB, Smith BD, Wittenborn JS, Lesesne SB, Wagner LD, Roblin DW, et al. The Cost-Effectiveness of Birth-Cohort Screening for Hepatitis C Antibody in U.S. Primary Care Settings. Ann Intern Med. 2012; 156:263-70.
Ross DB, Belperio PS, Chartier M, Backus LI. Hepatitis C testing in U.S. veterans born 1945–1965: An update. J Hepatol. 2017; 66:237-8.
Cabana MD, Rand CS, Powe NR, Wu AW, Wilson MH, Abboud PA, et al. Why don't physicians follow clinical practice guidelines? A framework for improvement. Jama. 1999; 282:1458-65.
Yakovchenko V, Bolton RE, Drainoni M-L, Gifford AL. Primary care provider perceptions and experiences of implementing hepatitis C virus birth cohort testing: a qualitative formative evaluation. BMC Health Serv Res 2019; 19:236.
Aarons GA. Transformational and transactional leadership: association with attitudes toward evidence-based practice. Psychiatr Serv 2006; 57:1162-9.
VA. VHA Handbook 1058.05, VHA Operations Activities That May Constitute Research. 2011.
Hoffmann TC, Glasziou PP, Boutron I, Milne R, Perera R, Moher D, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ 2014; 348:g1687.
Proctor EK, Powell BJ, McMillen JC. Implementation strategies: recommendations for specifying and reporting. Implement Sci 2013; 8:139.
Harvey G, Kitson A. PARIHS revisited: from heuristic to integrated framework for the successful implementation of knowledge into practice. Implement Sci 2016; 11:33.
Ritchie MJ KJ, Townsend JC, Pitcock JA, Dollar KM, Liu CF. Time and Organizational Cost for Facilitating Implementation of Primary Care Mental Health Integration. J Gen Intern Med. 2019.
Jacobs SR, Weiner, B.J. & Bunger, A.C. Context matters: measuring implementation climate among individuals and groups. Implement Sci. 2014; 9.
Helfrich CD, Li Y-F, Sharp ND, Sales AEJIS. Organizational readiness to change assessment (ORCA): development of an instrument based on the Promoting Action on Research in Health Services (PARIHS) framework. 2009; 4:38.
Helfrich CD, Li Y-F, Sharp ND, Sales AE. Organizational readiness to change assessment (ORCA): Development of an instrument based on the Promoting Action on Research in Health Services (PARIHS) framework. Implement Sci 2009; 4:38.
Jacobs SR, Weiner BJ, Bunger AC. Context matters: measuring implementation climate among individuals and groups. Implement Sci 2014; 9:46.
Noy C. Sampling Knowledge: The Hermeneutics of Snowball Sampling in Qualitative Research. Int J Soc Res Methodol 2008; 11:327-44.
Penfold RB, Zhang F. Use of interrupted time series analysis in evaluating health care quality improvements. Acad Pediatr 2013; 13:S38-44.
Elo S, Kyngas H. The qualitative content analysis process. J Adv Nurs 2008; 62:107-15.
Taylor B, Henshall C, Kenyon S, Litchfield I, Greenfield S. Can rapid approaches to qualitative analysis deliver timely, valid findings to clinical leaders? A mixed methods study comparing rapid and thematic analysis BMJ Open 2018; 8:e019993.
Sobo EJ, Simmes DR, Landsverk JA, Kurtin PS. Rapid Assessment with Qualitative Telephone Interviews: Lessons from an Evaluation of California’s Healthy Families Program & Medi-Cal for Children. Am J Eval 2003; 24:399-408.
Renz SM, Carrington JM, Badger TA. Two Strategies for Qualitative Content Analysis: An Intramethod Approach to Triangulation. Qual Health Res 2018; 28:824-31.
Korom-Djakovic D, Canamucio A, Lempa M, Yano EM, Long JA. Organization Complexity and Primary Care Providers' Perceptions of Quality Improvement Culture Within the Veterans Health Administration. Am J Med Qual 2016; 31:139-46.
Kirchner JE, Ritchie MJ, Pitcock JA, Parker LE, Curran GM, Fortney JC. Outcomes of a partnered facilitation strategy to implement primary care-mental health. J Gen Intern Med 2014; 29 Suppl 4:904-12.
Bidassie B, Williams LS, Woodward-Hagg H, Matthias MS, Damush TM. Key components of external facilitation in an acute stroke quality improvement collaborative in the Veterans Health Administration. Implement Sci 2015; 10:69.
Miech EJ, Rattray NA, Flanagan ME, Damschroder L, Schmid AA, Damush TM. Inside help: An integrative review of champions in healthcare-related implementation. SAGE Open Med 2018; 6:2050312118773261.
Janich N. Facilitator Withdrawal from Organizational Change Initiatives: A Review of Strategies and Guidelines. Group Facilitation. 2016.
van Bodegom-Vos L, Davidoff F, Marang-van de Mheen PJ. Implementation and de-implementation: two sides of the same coin? BMJ Qual Saf 2017; 26:495-501.
Colquhoun H, Michie S, Sales A, Ivers N, Grimshaw JM, Carroll K, et al. Reporting and design elements of audit and feedback interventions: a secondary review. BMJ Qual Saf 2017; 26:54-60.
Chambers DA, Glasgow RE, Stange KC. The dynamic sustainability framework: addressing the paradox of sustainment amid ongoing change. Implement Sci 2013; 8:117.
Proctor E, Luke D, Calhoun A, McMillen C, Brownson R, McCrary S, et al. Sustainability of evidence-based healthcare: research agenda, methodological advances, and infrastructure support. Implement Sci 2015; 10:88.
Shelton RC, Cooper BR, Stirman SW. The Sustainability of Evidence-Based Interventions and Practices in Public Health and Health Care. Annu Rev Public Health 2018; 39:55-76.
Goetz MB, Hoang T, Henry SR, Knapp H, Anaya HD, Gifford AL, et al. Evaluation of the sustainability of an intervention to increase HIV testing. J Gen Intern Med 2009; 24:1275-80.
CDC. Viral Hepatitis 2015 Surveillance Report. 2017.
Rosenberg ES, Rosenthal EM, Hall EW, Barker L, Hofmeister MG, Sullivan PS, et al. Prevalence of Hepatitis C Virus Infection in US States and the District of Columbia, 2013 to 2016. JAMA Netw Open 2018; 1:e186371.
Owens DK DK, Krist AH, Barry MJ, Cabana M, Caughey AB, Donahue K, Doubeni CA, Epling JW, Kubik M, Ogedegbe G. Screening for hepatitis C virus infection in adolescents and adults: US Preventive Services Task Force recommendation statement. JAMA. 2020:323(10):970-5.
Chhatwal J, Sussman NL. Universal Screening for Hepatitis C: An Important Step in Virus Elimination. Clin Gastroenterol Hepatol 2019; 17:835-7.
Funding
This study is funded by the Veterans Health Administration (VHA) Quality Enhancement Research Initiative (QUERI) Bridging the Care Continuum Program (QUE 15-284).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This project was conducted in accordance with VHA Handbook (1058.05) quality improvement guidelines and was deemed exempt from review by the VA Bedford VA Institutional Review Board.
Conflict of Interest
Authors declare that they do not have conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 16 kb)
Rights and permissions
About this article
Cite this article
Yakovchenko, V., DeSotto, K., Drainoni, ML. et al. Using Lean-Facilitation to Improve Quality of Hepatitis C Testing in Primary Care. J GEN INTERN MED 36, 349–357 (2021). https://doi.org/10.1007/s11606-020-06210-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11606-020-06210-5