Abstract
Background
Traditional Roux-en-Y may cause Roux-en-Y stasis syndrome (RSS), and Uncut Roux-en-Y was proposed to solve this problem. However, because afferent loop recanalization may occur after surgery, its clinical application remains controversial. The purpose of this study was to compare the long-term outcomes of these two gastrointestinal reconstruction methods.
Methods
A total of 108 patients who received laparoscopic-assisted distal gastrectomy (LADG) were enrolled; 57 were randomly divided into the Uncut Roux-en-Y (URY) group, and 51 were divided into the Roux-en-Y (RY) group. Patients were followed up for 1 year to evaluate variables, including the following: (1) Assessments for RSS; (2) Preoperative and postoperative Gastrointestinal Symptom Rating Scale (GSRS) scores; (3) Postoperative gastroscopy to assess the occurrence of reflux esophagitis (Los Angeles classification), residual gastritis and bile reflux 1 year after surgery; and (4) Upper gastrointestinal radiography to evaluate whether recanalization occurred in patients in the URY group after surgery.
Results
At 1 year after surgery, a total of 42 patients (73.7%) developed afferent loop recanalization. The incidence of RSS was not different between the two groups (OR, 1.301 [95% CI, 0.482 to 3.509]; P = 0.603P = 0.603). The GSRS score was higher in the URY group (P < 0.001). Postoperative gastroscopy showed that the incidence of bile reflux (P < 0.001) and the grade of residual gastritis (P < 0.001) were significantly higher in the URY group, but the grade of reflux esophagitis was not significantly different (P = 0.447, [95% CI, 0.437 to 0.457]P = 0.397).
Conclusions
Compared with traditional Roux-en-Y anastomosis, due to the high recanalization rate, the URY group developed more severe gastrointestinal symptoms, the incidence of bile reflux and the grade of residual gastritis increased and the incidence of postoperative RSS was not reduced.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The most common surgical approach for gastric antrum malignancies is radical gastrectomy. The standard surgical approach is distal gastrectomy (DG) combined with D2 lymphadenectomy.1,2 As technology advances, laparoscopy-assisted distal gastrectomy (LADG) has gained popularity for general surgeons because of better postoperative outcomes.3
Gastrointestinal anastomosis is essential for gastric cancer surgery. Compared with Billroth I and Billroth II, several studies have reported that Roux-en-Y can effectively reduce the incidence of residual gastritis and esophagitis,4,5,6,7,8 surgical complications,9,10 and the recurrence rate of gastric cancer.11 However, traditional Roux-en-Y anastomosis still has its own disadvantages; approximately 30% of patients have postoperative symptoms associated with RSS,12,13 including epigastric distention, nausea and vomiting.14 The main reasons related to the continuity of the jejunum were blockages and changes in small intestine electrical conduction.15,16 To further improve the surgical method, some scholars developed Uncut Roux-en-Y anastomosis; this technique blocks the afferent loop, which is approximately 3 cm from the gastrojejunostomy.17,18 However, according to previous studies, the afferent limb recanalization rate ranges from 2.9% to 35.7% after Uncut Roux-en-Y anastomosis.19,20
The purpose of this study was to investigate the recanalization rate of the afferent loop and compare these two anastomosis methods in terms of long-term results, including RSS, reflux esophagitis, bile reflux and residual gastritis.
Methods
Ethics
This was a prospective, two-center, two-arm randomized controlled trial (RCT). Ethics approval was obtained from Shanghai Tongji Hospital (number: 2018-LCYJ-005), and approval was given from another center as needed. All patients provided written informed consent. The registration number is ChiCTR-1800015228.
Patients
From June 2018 to December 2021, 140 adult patients who were scheduled to undergo laparoscopy-assisted distal gastrectomy (LADG) with D2 lymphadenectomy were recruited from Shanghai Tongji Hospital and Ningbo Hwa Mei Hospital. Twenty-three patients were excluded based on the exclusion criteria. The 117 included patients were randomly assigned (1:1) into two groups. Fifty-nine patients underwent Uncut Roux-en-Y surgery in the study group (URY group), and 58 patients underwent traditional Roux-en-Y surgery in the control group (RY group). During the postoperative follow-up, two patients died after discharge in the URY group, and 7 patients were lost to follow-up in the RY group. Ultimately, 57 patients were included in the URY group, and 51 patients were included in the RY group. All surgeons had surgical experience with at least 100 LADG surgeries.
The inclusion criteria were as follows: (1) patients aged ≥ 18 years; (2) patients with endoscopic biopsy and pathology reports confirming primary gastric cancer; (3) patients with stage I-III clinical tumors; (4) patients with ECOG scores of 0–1; and (5) patients with American Society of Anesthesiologists (ASA) grade I—III tumors. The exclusion criteria were as follows: (1) unstable angina, myocardial infarction, or cerebrovascular events within the past 6 months; (2) various serious mental diseases; (3) emergency surgery or pyloric obstruction; (4) previous history of upper abdominal surgery; (5) neoadjuvant chemoradiotherapy before surgery; and (6) other malignant tumors. The scheme of the study process is shown in Fig. 1.
Preoperative Management
All patients underwent gastroscopy, biopsy, and computed tomography (CT) to assess tumor characteristics. Other tests, including electrocardiogram, echocardiogram, and pulmonary function tests, were used to examine cardiopulmonary function. Patients fasted for 6 h and had no water for 2 h before surgery. General anesthesia was maintained by endotracheal intubation. Nasogastric and urinary catheters were placed before surgery.
Surgical Approach
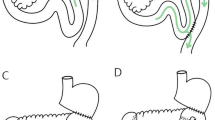
All enrolled patients underwent LADG combined with D2 lymphadenectomy. Roux-en-Y surgery was performed as described previously21. Uncut Roux-en-Y anastomosis was performed as follows: After closing the duodenal stump, side-to-side anastomosis of the stomach and jejunum was performed, and the distances with the ligament of Treitz were approximately 30 cm. Side-to-side anastomosis between the jejunum was performed approximately 35 cm from the gastrojejunostomy. Then, the afferent loop was blocked using 3 linear staplers with two rows of staples, and the length of the staplers used in the operation is 60 mm. (Fig. 2).
Postoperative Management
According to enhanced recovery after surgery (ERAS) principles,22 postoperative analgesia, early catheter removal, and early initiation of walking were performed on postoperative Day 1 (POD 1). The nasogastric tube was removed on POD 2. Patients were encouraged to eat a semisolid diet based on tolerance on POD 3. The drain was removed within 3 to 4 days.
Primary and Secondary Outcomes
The primary outcome was the incidence of RSS, which was assessed as follows: (1) Nausea, vomiting, and abdominal pain after surgery; (2) Refasting after return to a semifluid or normal diet; and (3) Readmission due to these reasons after surgery. Patients diagnosed with gastroparesis, intestinal paralysis, and anastomotic stenosis through clinical and image findings were not considered to have RSS.23 The secondary outcomes were preoperative and postoperative Gastrointestinal Symptom Rating Scale (GSRS) scores. Postoperative gastroscopy was used to assess the occurrence of the reflux esophagitis grade (Los Angeles classification), residual gastritis, and the degree of bile reflux at 1 year after surgery.
Gastroscope Grading
The gastroscopic esophagitis grade criteria followed the Los Angeles Classification System:24 (1) Grade A refers to one (or more) esophageal mucosal breaks less than 5 mm; (2) Grade B refers to one (or more) esophageal mucosal breaks greater than 5 mm; (3) Grade C refers to one (or more) mucosal breaks that are continuous between the tops of two or more mucosal folds but involves less than 75% of the circumference; and (4) Grade D refers to one (or more) mucosal breaks that involve more than 75% of the circumference.
Residual gastritis was divided into four categories: 0, the same as the surrounding normal tissue; I, local hyperemia and edema of the residual gastric mucosa; II, mucosa with scattered or intermittent linear hyperemia and edema; and III, widespread residual gastric mucosa hyperemia and edema.
Sample Size
The required sample size in each group was calculated using G*Power (University Kiel, Germany) software. There have been no exact incidences of RSS after RY or URY evaluated based on a large cohort. A minimum sample size of 44 patients per randomization arm was estimated to yield a statistical power of at least 0.8 with an alpha of 0.05 and a medium effect size (d = 0.3). Considering a loss to follow-up of up to 10%, at least 50 patients should be included in each group.
Statistical Methods
IBM SPSS Statistics 26 statistical software was used for analysis. Student’s t test was used to compare continuous variables with a normal distribution. Categorical variables were compared by the chi-square test, and P values < 0.05 were considered statistically significant.
Results
Patient Characteristics and Perioperative Surgical Outcomes
After screening and excluding patients who dropped out and were lost to follow-up, 57 patients were included in the URY group and 51 patients were included in the RY group. Clinical data are shown in Table 1. There were no significant differences in sex (OR, 0.548 [95%CI, 0.240 to 1.255]; P = 0.153), age (66.16 ± 7.94 vs 66.34 ± 9.05; P = 0.380), tumor location (OR, 0.646 [95%CI, 0.275 to 1.521]; P = 0.316), preoperative GSRS symptoms (P = 0.114, [95%CI, 0.112 to 0.125]), gastroesophageal reflux symptoms (OR, 0.463 [95%CI, 0.209 to 1.026]; P = 0.056), or clinicopathological stages (P = 0.424, [95%CI, 0.415 to 0.434]).
The difference in the conversion rate from laparoscopic to open surgery and the hospital stay (17.49 ± 6.84 vs 16.84 ± 6.04; P = 0.605) were not statistically significant. The URY group had a longer duration of surgery (219.89 ± 40.27 vs 195.12 ± 39.70, P = 0.002) and a higher incidence of surgical complications (P = 0.024, [95%CI, 0.021 to 0.027]), mainly Clavien‒Dindo grades I-II, such as poor gastroparesis (3 cases), poor incision healing (2 cases), intestinal paralysis (1 case), pancreatic fistula (1 case), and lymphatic fistula (1 case) (Table 2).
Recanalization Results
After a one-year follow-up, the cumulative number of recanalizations was 42 (73.7%) through upper gastrointestinal radiography (Fig. 3), and the recanalization of different periods is shown in Table 3. Upper gastrointestinal radiography after Uncut Roux-en-Y anastomosis was performed to evaluate whether patients were recanalized (Fig. 4).
Upper gastrointestinal radiography after Uncut Roux-en-Y anastomosis. Upper gastrointestinal radiography showing the closure of the afferent limb (black arrows) 6 months after the operation (a) and recanalization (black arrow) 9 months after the operation (b). In another case, upper gastrointestinal radiography showing the closure of the afferent limb (black arrow) 9 months after the operation (c) and recanalization (black arrow) 12 months after the operation (d)
Follow-Up Results
The postoperative follow-up results of the two groups are shown in Table 4. Nine patients (15.8%) in the URY group and 10 patients (19.6%) in the RY group developed RSS, and the incidence of RSS did not differ (OR, 1.301 [95%CI, 0.482 to 3.509]; P = 0.603). The GSRS score of the URY group was substantially higher than that of the RY group (P < 0.001).
Postoperative gastroscopy (Fig. 5) showed that there was no difference in reflux esophagitis grades (P = 0.447, [95%CI, 0.437 to 0.457]), but the grade of residual gastritis (P < 0.001) and the incidence of bile reflux (P < 0.001) in the URY group were significantly higher than those in the RY group.
Gastroscopy in the URY group at 1 year after surgery. (a) Endoscopy showing esophageal mucosal hyperemia and edema. (b) Endoscopy showing residual gastric hyperemia and edema with ulcers and bile reflux seen around. (c) and (d) Both showing recanalization of the afferent limb, bile reflux, yellow‒green mucus, and anastomotic edema
Subgroup Analysis
To explore the effect of recanalization on follow-up outcomes, patients in the URY group were assigned to the recanalization group (n = 42) and the nonrecanalization group (n = 15). The results are shown in Table 5. There was no difference in the grade of reflux esophagitis (P = 0.192, [95%CI, 0.184 to 0.200]), while the incidence of residual gastritis (P = 0.043, [95%CI, 0.046 to 0.054]) and the degree of bile reflux (OR, 4.800[95%CI, 1.370 to 16.812]; P = 0.011) were significantly higher in the recanalization group. No difference was observed in the incidence of RSS (OR, 0.304[95%CI, 0.035 to 2.659]; P = 0.474), but the GSRS score in the recanalization group was substantially higher than that in the RY group (P = 0.041, [95%CI, 0.037 to 0.044]).
Discussion
Uncut Roux-en-Y was invented to improve gastrointestinal reconstruction and reduce postoperative complications. However, in this trial, the incidence of RSS did not differ between the two groups. At the same time, the URY group showed a significantly higher incidence of residual gastritis and degree of bile reflux, and the GSRS score was obviously higher after surgery.
Uncut Roux-en-Y was invented by Van Stiegmann and Goff in 1988;25 this method does not cut off the jejunum, maintains the continuity of proximal jejunal anatomy and electrophysiological activity, avoids secondary pacemakers in the intestine, and helps relieve RSS symptoms.26,27 However, because afferent loop recanalization may occur after surgery, Uncut Roux-en-Y anastomosis has long been controversial. In our previous animal experiments with pigs, the incidence of recanalization reached 100% at 1 month after surgery.28 In this clinical study, the commonly used 6-row nails were used to close the afferent limb, and the recanalization rate was as high as 73.7%.
According to a previous study, closing the afferent loop jejunum may not result in permanent healing between the mucosa and the mucosa, which is the main reason for the recanalization of the bowel.29,30,31 This finding indicates that the linear stapler is not complete enough to close the afferent limb jejunum and needs to be further improved. Some scholars have proposed different methods of closing the afferent loop, and it has been reported that ligation with a 7-gauge silk thread can effectively reduce the recanalization rate. However, the strength of ligating the intestinal tube is not easy to grasp, and the ligation of the intestinal tube is too loose, which can lead to the occurrence of recanalization. There are also studies that improve this, with 6–8 layers of seromuscular layer in the upper and lower sections of the intestinal canal sutured at the closure, with the aim to further reduce the recanalization rate of the afferent limb.20,32,33
Generally, Uncut Roux-en-Y anastomosis involves Billroth II combined with Braun anastomosis, which prevents RSS after surgery.34 Many previous studies have analyzed the differences between Uncut Roux-en-Y and Billroth II and showed that Uncut Roux-en-Y can significantly decrease the incidence of residual gastritis and bile reflux.18,35,36 However, clinical trials of Uncut Roux-en-Y and Roux-en-Y are rarely reported. Our study found that the URY group did not show any advantages in preventing RSS. Moreover, the incidence of residual gastritis and the degree of bile reflux in the URY group were significantly higher than that in the RY group, which may be associated with the high recanalization rate, and our subgroup analysis also supports this conclusion.
Several limitations were identified in this study. First, the study design and sample size aimed to explore the advantages and disadvantages of these two reconstruction methods. When performing subgroup analysis, the results may not be sufficient for statistical analysis. Second, although many methods have been reported, blocking the afferent loop using a 6-row stapler is still the mainstream method.37 Therefore, this method was adopted in this study.
This study shows that the linear stapler cannot effectively block the recanalization of the afferent limb. Due to the high recanalization rate, the URY group developed more severe gastrointestinal symptoms, bile reflux, and had an increased residual gastritis incidence, and did not have a reduced incidence of postoperative RSS. Therefore, uncut Roux-en-Y reconstruction is not recommended before finding a way to reduce the recanalization rate.
Data Availability
The raw data of this paper are available from the authors upon reasonable request.
References
Smyth EC, Nilsson M and Grabsch HI, et al. Gastric cancer. Lancet 2020; 396: 635–648. Journal Article; Research Support, Non-U.S. Gov't; Review. https://doi.org/10.1016/S0140-6736(20)31288-5.
van de Cornelis Velde JH, Putter H and Kranenbarg EM, et al. Surgical treatment of gastric cancer: 15-year follow-up results of the randomised nationwide Dutch D1D2 trial. Lancet Oncol. 2010; 11: 439–449. Comparative Study; Journal Article; Randomized Controlled Trial; Research Support, Non-U.S. Gov't. https://doi.org/10.1016/S1470-2045(10)70070-X.
Kim H, Han S and Kim M, et al. Effect of Laparoscopic Distal Gastrectomy vs Open Distal Gastrectomy on Long-term Survival Among Patients With Stage I Gastric Cancer. Jama Oncol 2019; 5: 506. https://doi.org/10.1001/jamaoncol.2018.6727.
Chen X, Chen Y and Chen D, et al. The Development and Future of Digestive Tract Reconstruction after Distal Gastrectomy: A Systemic Review and Meta-Analysis. J. Cancer 2019; 10: 789-798. https://doi.org/10.7150/jca.28843.
Ren Z and Wang W. Comparison of Billroth I, Billroth II, and Roux-en-Y Reconstruction After Totally Laparoscopic Distal Gastrectomy: A Randomized Controlled Study. Adv. Ther. 2019; 36: 2997-3006. https://doi.org/10.1007/s12325-019-01104-3.
So JB, Rao J and Wong AS, et al. Roux-en-Y or Billroth II Reconstruction After Radical Distal Gastrectomy for Gastric Cancer. Ann. Surg. 2018; 267: 236-242. https://doi.org/10.1097/SLA.0000000000002229.
Collard JM and Romagnoli R. Roux-en-Y jejunal loop and bile reflux. Am. J. Surg. 2000; 179: 298–303. Comparative Study; Journal Article. https://doi.org/10.1016/s0002-9610(00)00326-3.
Zhu G, Hu J and Lu L, et al. A Comparison of the Short-Term Clinical Effects Between Totally Laparoscopic Radical Gastrectomy With Modified Roux-en-Y Anastomosis and Laparoscopic-Assisted Radical Gastrectomy With Roux-en-Y Anastomosis. Technol. Cancer Res. T. 2020; 19: 1180566096. https://doi.org/10.1177/1533033820973281.
Liu X, Gao Z and Wang R, et al. Comparison of Billroth I, Billroth II, and Roux-en-Y reconstructions after distal gastrectomy according to functional recovery: a meta-analysis. Eur Rev Med Pharmaco 2019; 23: 7532-7542. https://doi.org/10.26355/eurrev_201909_18869.
Wu C, Huang K and Chen M, et al. Comparison of the Long-term Outcome Between Billroth-I and Roux-en-Y Reconstruction Following Distal Gastrectomy for Gastric Cancer. J. Gastrointest. Surg. 2021; 25: 1955-1961. https://doi.org/10.1007/s11605-020-04867-1.
Cai Z, Zhou Y and Wang C, et al. Optimal reconstruction methods after distal gastrectomy for gastric cancer. Medicine 2018; 97: e10823. https://doi.org/10.1097/MD.0000000000010823.
Gustavsson S, Ilstrup DM and Morrison P, et al. Roux-Y stasis syndrome after gastrectomy. The American Journal of Surgery 1988; 155: 490-494. https://doi.org/10.1016/S0002-9610(88)80120-X.
Nakanishi K, Kanda M and Ito S, et al. Propensity-score-matched analysis of a multi-institutional dataset to compare postoperative complications between Billroth I and Roux-en-Y reconstructions after distal gastrectomy. Gastric Cancer 2020; 23: 734-745. https://doi.org/10.1007/s10120-020-01048-6.
Gustavsson S, Ilstrup DM and Morrison P, et al. Roux-Y stasis syndrome after gastrectomy. Am. J. Surg. 1988; 155: 490–494. Journal Article; Research Support, Non-U.S. Gov't; Research Support, U.S. Gov't, P.H.S. https://doi.org/10.1016/s0002-9610(88)80120-x.
Ishigami S, Natsugoe S and Hokita S, et al. Postoperative long-term evaluation of interposition reconstruction compared with Roux-en-Y after total gastrectomy in gastric cancer: prospective randomized controlled trial. The American Journal of Surgery 2011; 202: 247-253. https://doi.org/10.1016/j.amjsurg.2011.04.004.
Kumagai K, Hiki N and Nunobe S, et al. Different Features of Complications with Billroth-I and Roux-en-Y Reconstruction After Laparoscopy-Assisted Distal Gastrectomy. J. Gastrointest. Surg. 2011; 15: 2145-2152. https://doi.org/10.1007/s11605-011-1683-7.
Shibata C, Kobayashi T and Ueno T, et al. T1753 Results of Uncut Roux-En Y Reconstruction After Distal Gastrectomy for Gastric Cancer. Gastroenterology 2008; 134: 890-891. https://doi.org/10.1016/S0016-5085(08)64180-3.
Li Y, Wang Q and Yang K, et al. Uncut Roux-en-Y might reduce the rate of reflux gastritis after radical distal gastrectomy: An evidence mapping from a systematic review. Int J. Surg 2022; 97: 106184. https://doi.org/10.1016/j.ijsu.2021.106184.
Shibata C, Kakyo M and Kinouchi M, et al. Results of modified uncut Roux-en-Y reconstruction after distal gastrectomy for gastric cancer. Hepatogastroenterology 2013; 60: 1797–1799. Journal Article.
Tu BN, Sarr MG and Kelly KA. Early clinical results with the uncut Roux reconstruction after gastrectomy: limitations of the stapling technique. Am. J. Surg. 1995; 170: 262–264. Clinical Trial; Journal Article; Research Support, Non-U.S. Gov't; Research Support, U.S. Gov't, P.H.S. https://doi.org/10.1016/s0002-9610(05)80011-x.
Park YK, Yoon HM and Kim Y, et al. Laparoscopy-assisted versus Open D2 Distal Gastrectomy for Advanced Gastric Cancer. Ann. Surg. 2018; 267: 638-645. https://doi.org/10.1097/SLA.0000000000002168.
Mortensen K, Nilsson M and Slim K, et al. Consensus guidelines for enhanced recovery after gastrectomy. Brit. J. Surg. 2014; 101: 1209-1229. https://doi.org/10.1002/bjs.9582.
Mathias JR, Fernandez A and Sninsky CA, et al. Nausea, vomiting, and abdominal pain after Roux-en-Y anastomosis: motility of the jejunal limb. Gastroenterology 1985; 88: 101–107. Journal Article; Research Support, U.S. Gov't, Non-P.H.S.; Research Support, U.S. Gov't, P.H.S. https://doi.org/10.1016/s0016-5085(85)80140-2.
Lundell LR, Dent J and Bennett JR, et al. Endoscopic assessment of oesophagitis: clinical and functional correlates and further validation of the Los Angeles classification. Gut 1999; 45: 172–180. Journal Article; Research Support, Non-U.S. Gov't. https://doi.org/10.1136/gut.45.2.172.
Van Stiegmann G and Goff JS. An alternative to Roux-en-Y for treatment of bile reflux gastritis. Surg Gynecol Obstet 1988; 166: 69–70. Journal Article.
Zhang Y, Liu X and Xue D, et al. Myoelectric activity and motility of the Roux limb after cut or uncut Roux-en-Y gastrojejunostomy. World journal of gastroenterology : WJG 2006; 12: 7699-7704. https://doi.org/10.3748/wjg.v12.i47.7699.
Wang Y, Li Z and Shan F, et al. [Comparison of the safety and the costs between laparoscopic assisted or totally laparoscopic uncut Roux-en-Y and BillrothII(+Braun reconstruction--a single center prospective cohort study]. Zhonghua Wei Chang Wai Ke Za Zhi 2018; 21: 312–317. Journal Article.
Wu F, Ni Z and Diao H, et al. Recanalization in Uncut Roux-en-Y Reconstruction: An Animal Experiment and a Clinical Study. Frontiers in Surgery 2021; 8. https://doi.org/10.3389/fsurg.2021.644864.
Hangtian C, Huabing H and Tianhang L, et al. Isoperistaltic versus antiperistaltic uncut Roux-en-Y anastomosis after distal gastrectomy for gastric cancer: a propensity score matched analysis. Bmc Surg 2020; 20. https://doi.org/10.1186/s12893-020-00936-z.
Zhou W, Dong C and Zang Y, et al. Initial experience of single-incision plus one port left-side approach totally laparoscopic distal gastrectomy with uncut Roux-en-Y reconstruction. World J. Gastroentero. 2020; 26: 4669-4679. https://doi.org/10.3748/wjg.v26.i31.4669.
Park YS, Shin DJ and Son S, et al. Roux Stasis Syndrome and Gastric Food Stasis After Laparoscopic Distal Gastrectomy with Uncut Roux-en-Y Reconstruction in Gastric Cancer Patients: A Propensity Score Matching Analysis. World J. Surg. 2018; 42: 4022-4032. https://doi.org/10.1007/s00268-018-4715-6.
Tu BN and Kelly KA. Motility Disorders after Roux-en-Y Gastrojejunostomy. Obes. Surg. 1994; 4: 219–226. Journal Article. https://doi.org/10.1381/096089294765558412.
Zheng CH, Lu J and Huang CM, et al. Treatment of locally advanced gastric cancer with the XELOX program of neoadjuvantchemotherapy combined with laparoscopic surgery: the experience in China. Hepatogastroenterology 2014; 61: 1876–1882. Comparative Study; Journal Article; Research Support, Non-U.S. Gov't.
Huang Y, Wang S and Shi Y, et al. Uncut Roux-en-Y reconstruction after distal gastrectomy for gastric cancer. Expert Rev Gastroenterol Hepatol 2016; 10: 1341–1347. Journal Article; Review. https://doi.org/10.1080/17474124.2016.1248404.
Huang C, Huang R and Chen H, et al. Chromatin Accessibility Regulates Gene Expression and Correlates With Tumor-Infiltrating Immune Cells in Gastric Adenocarcinoma. Front Oncol 2021; 10. https://doi.org/10.3389/fonc.2020.609940.
Wang J, Wang Q and Dong J, et al. Total Laparoscopic Uncut Roux-en-Y for Radical Distal Gastrectomy: An Interim Analysis of a Randomized, Controlled, Clinical Trial. Ann. Surg. Oncol. 2021; 28: 90-96. https://doi.org/10.1245/s10434-020-08710-4.
Ahn S, Son S and Lee C, et al. Intracorporeal Uncut Roux-en-Y Gastrojejunostomy Reconstruction in Pure Single-Incision Laparoscopic Distal Gastrectomy for Early Gastric Cancer: Unaided Stapling Closure. J. Am. Coll. Surgeons 2014; 218: e17-e21. https://doi.org/10.1016/j.jamcollsurg.2013.09.009.
Funding
This study was funded by a grant from the Scientific Research Program of Shanghai Science and Technology Commission (Grant No. 201840357, 202040036, SKW2038, SKW1921,) and the Clinical Research and Cultivation Project of Shanghai Tongji Hospital ( Grant No. ITJ (ZD) 1804).
Author information
Authors and Affiliations
Contributions
QH designed this study. FW, QH, QC, LL and BG, as the main surgeon. HX wrote this manuscript. HX, CH and ZN as the main data recorders and analysts. LL and QH revised the manuscript critically. HX and SW completed the follow up. All authors approved the submitted final version.
Corresponding authors
Ethics declarations
Disclosures
There are no financial conflicts of interest to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This was a prospective, two center, randomized controlled trial, open between June 2018 and December 2021.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Xie, H., Wu, F., Huang, C. et al. Tranditional Roux-en-Y vs Uncut Roux-en-Y in Laparoscopic Distal Gastrectomy: a Randomized Controlled Study. J Gastrointest Surg 27, 1098–1105 (2023). https://doi.org/10.1007/s11605-023-05644-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-023-05644-6