Abstract
Background
Liver venous deprivation (LVD) is a recent radiological technique performed to induce hypertrophy of the future liver remnant. Medium-term results of major hepatectomy after LVD have never been compared with the actual standard of care, portal vein embolization (PVE).
Methods
We retrospectively compared data from 33 consecutive patients who had undergone LVD (n = 17) or PVE (n = 16) prior to a right hemi-hepatectomy or right extended hepatectomy indicated for colorectal liver metastases (CRLM) between May 2015 and December 2019.
Results
The 1-year and 3-year overall survival (OS) rates in the LVD group were 81.3% (95% confidence interval [CI]: 72–90) and 54.7% (95% CI: 46–63), respectively, against 85% (95% CI: 69–101) and 77.4% (95% CI: 54–100) in the PVE group; the differences were not statistically significant (p = 0.64). The median disease-free survival (DFS) rate was also comparable: 6 months (95% CI: 4–7) in the LVD group and 12 months (95% CI: 1.5–13) in the PVE group (p = 0.29). The overall intra-operative and post-operative complication rates were similar between the two groups. The mean daily kinetic growth rate (KGR) was found to be higher after LVD than after PVE (0.2% vs. 0.1%, p = 0.05; 10 cc/day vs. 4.8 cc/day, p = 0.03), as was the mean increase in future liver remnant volume (FLR-V) (49% vs. 27%, p = 0.01).
Conclusions
The LVD technique is well tolerated in patients undergoing right hemi-hepatectomy or right extended hepatectomy for CRLM. When compared with the PVE technique, the LVD technique has similar peri-operative and medium-term outcomes, but higher KGR and FLR-V increase.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Colorectal cancer (CRC) is the third most frequent cause of cancer death worldwide, and approximately 25–30% of patients diagnosed with CRC develop liver metastases.1,2 Liver resection is considered the cornerstone treatment for colorectal liver metastases (CRLM), achieving 5-year survival rates higher than 50%, showing low morbidity and mortality in highly experienced centers.3,4 With the evolutions in medicine and surgery, the indications for the surgical treatment of CRLM have expanded in recent years.5 Unfortunately, until now, only about 25% of all CRLM patients are susceptible to undergo resection.6 Though parenchymal-sparing hepatectomy (PSH) is considered the standard of care strategy for CRLM, many patients need to undergo major hepatectomy because of their large tumor size or the relationship between the tumor and the main vascular structures.7 Owing to inadequate future liver remnant volume (FLR-V), less than 25% of patients are eligible for major hepatectomy at the time of cancer diagnosis.8 This is because a major hepatectomy can cause post-hepatectomy liver failure (PHLF), which is the leading cause of death after the resection of three or more liver segments.9
Therefore, to minimize the risk of PHLF, sufficient FLR-V must therefore be preserved.10 It is generally accepted that PHLF is likely to occur in patients whose FLR-V is less than 25% of the total volume of a normal liver or 30% of a fatty liver, who have had multiple courses of chemotherapy or who have 40% of a liver with cholestasis or cirrhosis.11 To optimize the FLR, several techniques have been developed to induce liver hypertrophy. Since its introduction in 1984 by Makuuchi et al., portal vein embolization (PVE) has been considered the standard technique for inducing FLR increase.12,13 However, PVE does not always induce sufficient and rapid hypertrophy: Up to 20% of treated patients are still unfit for completion surgery after a relatively long time (4–6 weeks) following the procedure due to insufficient FLR increase or tumor progression.14
To overcome these limitations, a new interventional radiological technique was described in 2016: hepatic venous deprivation (LVD).15 It consists of the simultaneous embolization of the portal vein and one or two hepatic veins (extended liver venous deprivation) in order to increase the damage to the contralateral liver and further induce hypertrophy of the FLR (approximate kinetic growth rate = 16 ± 7 cc/day, according to the initial reports).16,17 The first comparative data regarding the volume and functional increase of FLR were recently published, showing a greater regeneration after LVD than after PVE.18 The impact of LVD on hepatic recurrence (HR) and medium-term results after CLRM resection remains unclear. Furthermore, no studies have evaluated the early intra- and post-operative results after right hemi-hepatectomy and right trisectionectomy in CRLM patients in this setting. This retrospective study was therefore carried out to compare the short- and medium-term outcomes of major hepatectomy after LVD with those after PVE.
Materials and Methods
Study Design
This was a single-institution retrospective study conducted according to the Strengthening and Reporting of Observational Studies in Epidemiology (STROBE) guidelines of the EQUATOR network.19 Informed consent was obtained prior to the radiological and surgical procedures. This study was approved by the Institutional Ethics Committee of the University Hospital of Montpellier (IRB-MTP_2020_04_202000444).
Patients
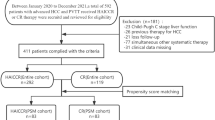
Data from patients who underwent consecutive LVD prior to right hemi-hepatectomy or right extended hepatectomy (trisectionectomy) for colorectal cancer metastases at the University Hospital of Montpellier between May 2015 and December 2019 were retrospectively collected and analyzed. Patients with liver cirrhosis were excluded from this study. The flowchart of the study is shown in Fig. 1. The choice of the therapeutic management of each of the included patients was taken after a previous discussion during a multidisciplinary oncology meeting. The decision to perform a liver augmentation procedure was based on the FLR volume and/or functional assessment using mebrophenin Tc-99 m scintigraphy. The radiological procedure was performed when the expected FLR was < 25% in the normal liver, < 30% in the liver that had undergone chemotherapy, or < 40% in cases of underlying liver disease (cholestasis), and when the Tc99m mebrophenin extraction was < 2.69%/min/m2. When both the volume and function of the FLR were insufficient, or when liver scintigraphy was not available, LVD was performed instead of PVE alone due to the fact that LVD showed a greater volumetric increase. Patient follow-up after LVD was based on contrast computed tomography (CT) and Tc99m-mebrofenin scintigraphy performed weekly after the procedure. Radiological and surgical procedures, as well as patient management, were performed by the same team in the same facility.
To compare the results obtained with LVD, data were retrospectively collected from consecutive patients who underwent the same type of liver resection for CRLM after PVE during the same time period.
Radiological Procedure
The LVD technique has previously been described in detail.15 Summarily, the right hepatic vein (and accessory vein, when present) was cannulated through trans-hepatic access under ultrasound guidance using a B1-stick technique.20 Initial PVE was performed through right trans-hepatic access. The right portal vessels were embolized using n-butyl cyanoacrylate and lipiodol (ratio 1:6). The micro guidewire placed in the hepatic vein(s) was then used to position an Amplatzer II vascular plug (75% opening). The plug was positioned at a distance of approximately 2 cm from the ostium of the inferior vena cava (IVC) to reduce the risk of plug overlength. Finally, all distal venous branches were embolized using a mixture of n-butyl cyanoacrylate and lipiodol (ratio 1:6). Eight patients also underwent embolization of the middle hepatic vein, a procedure called extended liver deprivation (eLVD). The decision to embolize the middle hepatic vein was made by the radiologist based on the size of the FLR, type of surgery, and anatomical characteristics of the hepatic vein circulation.
Surgical Intervention
Patients either underwent laparotomic right hemi-hepatectomy (segments 5–8), according to the Brisbane classification of liver resections, or extended right hepatectomy (trisectorectomy).21 An intra-operative ultrasound was performed to confirm the surgical feasibility of the procedure and to guide the resection. The right hepatic artery and portal vein were systematically ligated and dissected before parenchymal sectioning using an anterior approach. The hepatic veins were closed and divided using a vascular stapler; the Amplatzer-type plug did not prove to be an obstacle in this regard. If necessary, a Pringle maneuver with intermittent clamping or selective right portal vein control was performed.
Post-Operative Follow-up
Post-operative follow-up data were analyzed. Post-operative complications were classified according to the Clavien–Dindo classification.22 PHLF, post-hepatectomy bile leakage (PHBL), and post-hepatectomy hemorrhage (PHH) were diagnosed and classified according to the International Study Group of Liver Surgery (ISGLS) guidelines.23,24,25 Ascites was defined following the International Ascites Club definition.26 Synchronous metastases were defined, according to the Expert Group on OncoSurgery Management of Liver Metastases group (EGOSLIM) definition, as metastases detected by pre-operative screening or during resection of the primary tumor.27
All patients were examined within one month after discharge from the surgery department and underwent clinical, biological, and imaging evaluations every 3 months after discharge for the first two years, according to the oncological protocols. Outpatients’ controls were scheduled every 12 months if no relapse was found. In case of tumor recurrence, the case was re-examined by a multidisciplinary team (MDT) with the aim of carrying out curative treatment as much as possible.
Hypertrophy Parameters
The volume share of the FLR (FLR-V%) was calculated from manual reconstruction using the formula described by Vauthey et al.28: FLR-V share = FLR-V/(eTLV − TV) × 100, where eTLV = –794.41 + 1267.28 × body surface area. To compare hypertrophy responses, the kinetic growth rate (KGR) was calculated as the percentage growth per day [degree of hypertrophy (DH) at the first post-procedural volume assessment (%)/elapsed interval from the radiological procedure (days)], as well as in volume (cc) growth per day, that is, (FLR-V after intervention – FLR-V prior to intervention)/time elapsed.29 The degree of hypertrophy was calculated using the following formula30,31:
The increase in the FRL-V% was calculated using the following formula:
Volumes were compared with the last imaging exam performed before surgery.
Statistical Analysis and Endpoints
Continuous data were expressed as mean and standard deviation (SD) or median and interquartile range (IQR), depending on whether they had a normal distribution or not. Group comparisons were performed using Student’s T test or Wilcoxon’s rank test, depending on the distribution of the variable. Categorical data were expressed as frequencies and associated percentages. Comparisons between groups were performed using Pearson’s chi-squared test or Fisher’s exact test, depending on the expected value of the variable of interest.32 The primary endpoints were the overall survival (OS) and disease-free survival (DFS) rates. Secondary endpoints were peri-operative complications. All survival analyses were performed using the Kaplan–Meier method to calculate the median and 95% confidence interval (CI), and comparisons were performed using the log-rank method. The median follow-up was analyzed using the inverse Kaplan–Meier method. Statistical analysis was conducted using the software Statistical Package for Social Sciences (SPSS) software (version 26.0).
Results
Patients’ Characteristics
We retrospectively reviewed data from 17 consecutive patients who underwent LVD and 16 who underwent PVE prior to right hemi-hepatectomy or right extended hepatectomy (trisectionectomy) for CRLM. The mean age of the patients was 58.9 years (± 9.6). All LVD patients underwent chemotherapy (CT) prior to the liver surgery, against only 12 PVE patients. Among the LVD patients, 13 (77%) received neoadjuvant CT and 4 (23%) underwent a conversion surgery. Table 1 lists the different CT schemes used and the responses of the patients to the schemes. Fourteen patients additionally received post-operative CT (82.3%). No statistically significant differences were found in the pre-operative characteristics of the two groups. The patients’ and tumor characteristics are shown in Table 1. Regarding patients who underwent PVE, four (25%) had undergone previous hepatic surgery and two (12.5%) had undergone thermal ablation. The overall median follow-up period was 26 months (95% CI: 17–29).
Survival Analysis
The 1-year and 3-year overall survival (OS) rates were respectively 81.3% (95% CI: 72–90) and 54.7% (95% CI: 46–63) in the LVD group, and 85% (95% CI: 69–100) and 77.4% (95% CI: 54–99) in the PVE group (Fig. 2B). There were no statistically significant differences between the two populations (p = 0.64). The median disease-free survival (DFS) rates in the LVD group and PVE group were 6 months (95% CI: 4–7) and 12 months (CI 95%: 1.5–13), respectively. The 1-year DFS rates in the two groups were 53% (95% CI: 39–67) and 6.3% (95% CI: 0.3–12.3), respectively; meanwhile the 3-year DFS rates were 44% (95% CI: 30–58) and 0 (Fig. 2A). There were no statistically significant differences between the two populations (p = 0.29).
Hepatic recurrence occurred in nine patients (52.9%) in the LVD group and five patients in the PVE group (31.2%). In the LVD group, two patients developed pulmonary progression, two developed lumbo-aortic lymph node metastasis, and one developed peritoneal carcinosis. Six patients died during the follow-up period; however, the causes of the deaths were not related to post-operative or post-procedural events. Among the patients, four experienced pulmonary progression.
Secondary Endpoints
The median time between the LVD procedure and surgery was 39 days (IQR25-75: 25–56). No patient experienced serious complications after the radiological procedure, and all 17 patients underwent surgery after LVD. The successful resection rate for the LVD procedure was 100%. Regarding intra-operative outcomes, no statistically significant differences were observed between the two groups. The mean duration of the intervention was 327 min (± 93) in PVE patients and 288 min (± 62) in LVD patients. Both the estimated amount of intra-operative blood loss and the number of blood transfusions were comparable between the two groups (median, 500 vs. 700 cc and 1 vs. 0, respectively). Post-operative complications occurred in eight patients (47%) in the LVD group and eight patients as in the PVE group (50%). Following the Clavien–Dindo classification of complications, in the LVD group, two patients had grade I complications, five had grade II complications, and one had a grade III complication, requiring endoscopic retrograde cholangiopancreatography (ERCP) with sphincterotomy. In particular, seven patients had post-hepatectomy hemorrhage (41%), all of grade A according to ISGLS; one patient experienced a bile leakage (5%); three patients developed grade A PHLF (17%); and three patients developed post-operative grade A ascites (23.5%). It should be noted that the incidence of post-hepatectomy hemorrhage was significantly higher in LVD patients (p = 0.04), although they were all grade I complications. Conversely, the number of serious complications (defined as belonging to at least grade III of the Clavien–Dindo classification) was higher in the group of PVE patients (4 vs. 1, p = 0.07). The results of the univariate analysis of intra-operative and post-operative complications are shown in Table 2.
Volume Analysis
The median pre-procedural tumor volumes were similar in the PVE and LVD groups (100 cc vs. 51 cc, respectively, p = 0.24). The mean FLR-V was 32.2 before PVE and 29.3 before LVD (p = 0.53). However, the mean increase in FLR-V was higher after LVD (49% vs. 27%, p = 0.01). The mean daily KGR was also significantly higher after the LVD procedure (0.2% vs. 0.1%, p = 0.05; 10 cc/day vs. 4.8 cc/day, p = 0.03). All volume analysis data are listed in Table 3.
Discussion
To the best of our knowledge, this is the first comparative study on the short- and medium-term outcomes of right hemi-hepatectomy or right extended hepatectomy after LVD vs. after PVE, specifically for CRLM. Early intra- and post-operative results after LVD are very interesting; no differences were found between the PHLF rates, operative durations, estimated intra-operative blood loss, and biliary leak rates. It is interesting to note, however, that there was a greater number of post-operative bleeding events in patients who underwent LVD prior to surgery, even though all the cases were classified as minor bleeding. This finding could, in theory, be linked to the hemodynamic changes induced by the technique itself: Compared to the PVE, it induces a higher blood flow in the contralateral liver by closing also the hepatic veins. Therefore, it would be interesting in the future to further investigate this aspect as well as validate this finding by carrying out studies with larger samples and more appropriate designs. Our group has published the first comparative study investigating peri-operative outcomes among LVD and PVE patients; however, we did not focus on a specific surgical procedure or on a specific disease (such as hepatocellular carcinomas and cholangiocarcinomas).33 It is well known that including cirrhotic or cholestatic livers, as in the aforementioned study, can negatively affect the homogeneity of the sample and the power of the results. Furthermore, the real role of LVD in patients with cirrhosis remains debatable.34
Regarding the rate of severe post-operative complications, only one Clavien–Dindo grade III event was registered in the LVD group against four events in the PVE group, though the difference was not statistically significant. Furthermore, a greater proportion of patients in the PVE group had previously undergone surgery or thermal ablation; this could have influenced the results. Further comparative studies are required to clarify this finding. Similarly, the preliminary oncological outcomes of LVD in CRLM patients appear to be encouraging, as recently suggested in other studies.35,36 Previous studies have hypothesized negative effects on tumor growth following liver volume augmentation procedures, including PVE.37 However, a recent meta-analysis by Giglio et al. concluded that PVE did not adversely affect cancer outcomes after major hepatectomy in patients with CRLM.38 Furthermore, LVD has some technical peculiarities that could theoretically have other oncological implications (such as blocking the venous return of the diseased liver using Amplatzer-type plugs); however, these deserve further investigations. Herein, no significant differences in the incidence of hepatic recurrence and medium-term OS and DFS rates were found between the PVE and LVD groups.
Regarding volume analysis, the mean daily KGR was proven to be significantly higher after LVD than after PVE (0.2% vs. 0.1%, p = 0.05; 10 cc/day vs. 4.8 cc/day, p = 0.03). Similarly, the mean FLR-V increase was higher after LVD (49% vs. 27%, p = 0.01).36,39 These results are in line with previous reports,16,39 but need to be confirmed by larger randomized studies, such as the ongoing DRAGON-1 (NCT04272931) or Hyper-LIV01 (NCT03841305) studies. Furthermore, the mean weekly KGR was lower than 2% in the studied population (1.45 after LVD vs. 1.12 after PVE, p = 0.46): A result that was previously reported to be at risk of PHLF after PVE.31 However, we experienced only 3 cases of PHLF after PVE, and all of them were graded A according to ISGLS.
Another important concern arising from our analysis was the time lapse between the LVD procedure and the surgery (39 days). Previous studies have reported that delayed hepatic hypertrophy following PVE may itself be a major cause of cancer recurrences reported during FLR augmentation procedures.40 Similarly, the importance of starting post-operative chemotherapy as soon as possible after surgery is well known, and it is also indirectly correlated with the time to FLR regeneration during the post-operative course. Our data showed a median time between LVD and surgery of 39 days (IQR25–75, 25–56), which is comparable to several PVE reports in the literature.41 Nonetheless, this delay is too long for the procedure to induce faster hypertrophy of the FLR. However, several authors have proposed that this delay could be greatly reduced, as it has been shown that an increase in FLR volume and function occurs as early as seven days after LVD.39 One of the main reasons for the long time delay experienced by the patients examined in this study could be the fact that the LVD technique was just recently introduced; besides, it is being evaluated using the standard of previous studies. That is, volume measurements are performed on days 7 and 21, with the assumption that the growth effect is similar to that of the actual radiological standard of care (the longer the waiting time, the greater the FLR augmentation). As earlier suggested, previous findings encourage a significant reduction of this delay and the application of an ALPPS-like strategy.42 Further studies should focus on the reduction of the time delay when performing LVD procedures to ensure excellent patient tolerance and volumetric growth rate.43 More consistent efforts should also be made to reduce the time of surgery planning, though this is difficult in daily practice given the context of the COVID-19 pandemic characterized by fewer available resources. Finally, the successful resection rate after the LVD was 100%, concurring with a recent study involving 21 patients performed by Kobayashi et al.30 No case of death secondary to post-procedural complications or tumor progression was registered.
This study had several limitations. First, its observational and retrospective design, with purely univariate inferential statistics, was due to the small sample size and the number of variables. Because of the recent nature of this technique, few consecutive patients were included in this study; thus, the small sample size could not allow a wider and more detailed analysis of the factors that may play a prognostic role in both OS and DFS. Similarly, the median follow-up time was too short to adequately assess long-term outcomes: An update of the survival data will be needed in the coming years to assess the results after a longer follow-up. Nevertheless, all the oncological and non-oncological results are very encouraging and deserve to be shared to arouse interest and enthusiasm on this still a highly debated topic. Nonetheless, our study had several strengths. A rigorous selection of inclusion and exclusion criteria (with the exclusion of cirrhotic and cholestatic livers) limited our sample size but increased the homogeneity of the sample and the power of these results. Finally, to the best of our knowledge, this is the first study to compare the medium-term outcomes after LVD and PVE in patients undergoing right hepatectomy or trisectionectomy.
Conclusion
The LVD technique is well tolerated in patients undergoing right hemi-hepatectomy or right extended hepatectomy for CRLM, showing similar peri-operative and medium-term outcomes compared to PVE. It will be important to update the oncological data in the coming years to obtain the results after 5 years of follow-up, with the possible inclusion of new patients. Randomized controlled trials (RCTs) are needed to confirm the benefits of LVD.
References
Araghi M, Arnold M, Rutherford MJ, et al. Colon and rectal cancer survival in seven high-income countries 2010–2014: variation by age and stage at diagnosis (the ICBP SURVMARK-2 project). Gut. 2021;70(1):114-126. https://doi.org/10.1136/gutjnl-2020-320625
Hackl C, Neumann P, Gerken M, Loss M, Klinkhammer-Schalke M, Schlitt HJ. Treatment of colorectal liver metastases in Germany: a ten-year population-based analysis of 5772 cases of primary colorectal adenocarcinoma. BMC Cancer. 2014;14:810. https://doi.org/10.1186/1471-2407-14-810
House MG, Ito H, Gönen M, et al. Survival after hepatic resection for metastatic colorectal cancer: trends in outcomes for 1,600 patients during two decades at a single institution. J Am Coll Surg. 2010;210(5):744-752, 752-755. https://doi.org/10.1016/j.jamcollsurg.2009.12.040
Choti MA, Sitzmann JV, Tiburi MF, et al. Trends in long-term survival following liver resection for hepatic colorectal metastases. Ann Surg. 2002;235(6):759-766. https://doi.org/10.1097/00000658-200206000-00002
Van Cutsem E, Cervantes A, Adam R, et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol Off J Eur Soc Med Oncol. 2016;27(8):1386-1422. https://doi.org/10.1093/annonc/mdw235
Cassese G, Han HS, Al Farai A, Guiu B, Troisi RI, Panaro F. Future remnant Liver optimization: preoperative assessment, volume augmentation procedures and management of PVE failure. Minerva Surg. Published online March 25, 2022. https://doi.org/10.23736/S2724-5691.22.09541-7
Deng G, Li H, Jia GQ, et al. Parenchymal-sparing versus extended hepatectomy for colorectal liver metastases: a systematic review and meta-analysis. Cancer Med. 2019;8(14):6165-6175. https://doi.org/10.1002/cam4.2515
Khatri VP, Petrelli NJ, Belghiti J. Extending the frontiers of surgical therapy for hepatic colorectal metastases: is there a limit? J Clin Oncol Off J Am Soc Clin Oncol. 2005;23(33):8490-8499. https://doi.org/10.1200/JCO.2004.00.6155
Xu F, Tang B, Jin TQ, Dai CL. Current status of surgical treatment of colorectal liver metastases. World J Clin Cases. 2018;6(14):716-734. https://doi.org/10.12998/wjcc.v6.i14.716
Schindl MJ, Redhead DN, Fearon KCH, Garden OJ, Wigmore SJ, Edinburgh Liver Surgery and Transplantation Experimental Research Group (eLISTER). The value of residual liver volume as a predictor of hepatic dysfunction and infection after major liver resection. Gut. 2005;54(2):289-296. https://doi.org/10.1136/gut.2004.046524
Clavien PA, Petrowsky H, DeOliveira ML, Graf R. Strategies for safer liver surgery and partial liver transplantation. N Engl J Med. 2007;356(15):1545-1559. https://doi.org/10.1056/NEJMra065156
Makuuchi M, Takayasu K, Takuma T, et al. Preoperative transcatheter embolization of the portal venous branch for patients receiving extended lobectomy due to the bile duct carcinoma. J Jpn Pract Surg Soc. 1984;45(12):1558-1564. https://doi.org/10.3919/ringe1963.45.1558
Aoki T, Kubota K. Preoperative portal vein embolization for hepatocellular carcinoma: consensus and controversy. World J Hepatol. 2016;8(9):439-445. https://doi.org/10.4254/wjh.v8.i9.439
Alvarez FA, Castaing D, Figueroa R, et al. Natural history of portal vein embolization before liver resection: a 23-year analysis of intention-to-treat results. Surgery. 2018;163(6):1257-1263. https://doi.org/10.1016/j.surg.2017.12.027
Guiu B, Chevallier P, Denys A, et al. Simultaneous trans-hepatic portal and hepatic vein embolization before major hepatectomy: the liver venous deprivation technique. Eur Radiol. 2016;26(12):4259-4267. https://doi.org/10.1007/s00330-016-4291-9
Guiu B, Quenet F, Escal L, et al. Extended liver venous deprivation before major hepatectomy induces marked and very rapid increase in future liver remnant function. Eur Radiol. 2017;27(8):3343-3352. https://doi.org/10.1007/s00330-017-4744-9
Memeo R, Conticchio M, Deshayes E, et al. Optimization of the future remnant liver: review of the current strategies in Europe. Hepatobiliary Surg Nutr. 2021;10(3):350-363. https://doi.org/10.21037/hbsn-20-394
Deshayes E, Schadde E, Piron L, Quenet F, Guiu B. Extended liver venous deprivation leads to a higher increase in liver function that ALPPS in early assessment : a comment to “Sparrelid, E. et al. Dynamic evaluation of liver volume and function in associating liver partition and portal vein ligation for staged hepatectomy. Journal of Gastrointestinal Surgery (2017).” J Gastrointest Surg Off J Soc Surg Aliment Tract. 2017;21(10):1754-1755. https://doi.org/10.1007/s11605-017-3508-9
Cuschieri S. The STROBE guidelines. Saudi J Anaesth. 2019;13(Suppl 1):S31-S34. https://doi.org/10.4103/sja.SJA_543_18
Pomerantz BJ. Biliary tract interventions. Tech Vasc Interv Radiol. 2009;12(2):162-170. https://doi.org/10.1053/j.tvir.2009.08.009
The Brisbane 2000 Terminology of liver anatomy and resections. HPB 2000; 2:333–39. HPB. 2002;4(2):99-100. https://doi.org/10.1080/136518202760378489
Clavien PA, Barkun J, de Oliveira ML, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250(2):187-196. https://doi.org/10.1097/SLA.0b013e3181b13ca2
Rahbari NN, Garden OJ, Padbury R, et al. Posthepatectomy liver failure: a definition and grading by the International Study Group of Liver Surgery (ISGLS). Surgery. 2011;149(5):713-724. https://doi.org/10.1016/j.surg.2010.10.001
Rahbari NN, Garden OJ, Padbury R, et al. Post-hepatectomy haemorrhage: a definition and grading by the International Study Group of Liver Surgery (ISGLS). HPB. 2011;13(8):528-535. https://doi.org/10.1111/j.1477-2574.2011.00319.x
Koch M, Garden OJ, Padbury R, et al. Bile leakage after hepatobiliary and pancreatic surgery: a definition and grading of severity by the International Study Group of Liver Surgery. Surgery. 2011;149(5):680-688. https://doi.org/10.1016/j.surg.2010.12.002
Moore KP, Wong F, Gines P, et al. The management of ascites in cirrhosis: report on the consensus conference of the International Ascites Club. Hepatol Baltim Md. 2003;38(1):258-266. https://doi.org/10.1053/jhep.2003.50315
Adam R, de Gramont A, Figueras J, et al. Managing synchronous liver metastases from colorectal cancer: a multidisciplinary international consensus. Cancer Treat Rev. 2015;41(9):729-741. https://doi.org/10.1016/j.ctrv.2015.06.006
Vauthey JN, Abdalla EK, Doherty DA, et al. Body surface area and body weight predict total liver volume in Western adults. Liver Transplant Off Publ Am Assoc Study Liver Dis Int Liver Transplant Soc. 2002;8(3):233-240. https://doi.org/10.1053/jlts.2002.31654
Croome KP, Hernandez-Alejandro R, Parker M, Heimbach J, Rosen C, Nagorney DM. Is the liver kinetic growth rate in ALPPS unprecedented when compared with PVE and living donor liver transplant? A multicentre analysis. HPB. 2015;17(6):477-484. https://doi.org/10.1111/hpb.12386
Kobayashi K, Yamaguchi T, Denys A, et al. Liver venous deprivation compared to portal vein embolization to induce hypertrophy of the future liver remnant before major hepatectomy: a single center experience. Surgery. 2020;167(6):917-923. https://doi.org/10.1016/j.surg.2019.12.006
Shindoh J, Truty MJ, Aloia TA, et al. Kinetic growth rate after portal vein embolization predicts posthepatectomy outcomes: toward zero liver-related mortality in patients with colorectal liver metastases and small future liver remnant. J Am Coll Surg. 2013;216(2):201-209. https://doi.org/10.1016/j.jamcollsurg.2012.10.018
Jung SH. Stratified Fisher’s exact test and its sample size calculation. Biom J Biom Z. 2014;56(1):129-140. https://doi.org/10.1002/bimj.201300048
Panaro F, Giannone F, Riviere B, et al. Perioperative impact of liver venous deprivation compared with portal venous embolization in patients undergoing right hepatectomy: preliminary results from the pioneer center. Hepatobiliary Surg Nutr. 2019;8(4):329-337. https://doi.org/10.21037/hbsn.2019.07.06
Guiu B, Herrero A, Panaro F. Liver venous deprivation: a bright future for liver metastases—but what about hepatocellular carcinoma? Hepatobiliary Surg Nutr. 2021;10(2):270-272. https://doi.org/10.21037/hbsn-21-7
Khayat S, Cassese G, Quenet F, et al. Oncological outcomes after liver venous deprivation for colorectal liver metastases: a single center experience. Cancers. 2021;13(2):E200. https://doi.org/10.3390/cancers13020200
Cassese G, Troisi RI, Khayat S, et al. Liver venous deprivation versus associating liver partition and portal vein ligation for staged hepatectomy for colo-rectal liver metastases: a comparison of early and late kinetic growth rates, and perioperative and oncological outcomes. Surg Oncol. 2022;43:101812. https://doi.org/10.1016/j.suronc.2022.101812
Simoneau E, Aljiffry M, Salman A, et al. Portal vein embolization stimulates tumour growth in patients with colorectal cancer liver metastases. HPB. 2012;14(7):461-468. https://doi.org/10.1111/j.1477-2574.2012.00476.x
Giglio MC, Giakoustidis A, Draz A, et al. Oncological outcomes of major liver resection following portal vein embolization: a systematic review and meta-analysis. Ann Surg Oncol. 2016;23(11). https://doi.org/10.1245/s10434-016-5264-6
Guiu B, Quenet F, Panaro F, et al. Liver venous deprivation versus portal vein embolization before major hepatectomy: future liver remnant volumetric and functional changes. Hepatobiliary Surg Nutr. 2020;9(5):564-576. https://doi.org/10.21037/hbsn.2020.02.06
Beal IK, Anthony S, Papadopoulou A, et al. Portal vein embolisation prior to hepatic resection for colorectal liver metastases and the effects of periprocedure chemotherapy. Br J Radiol. 2006;79(942):473-478. https://doi.org/10.1259/bjr/29855825
van Lienden KP, van den Esschert JW, de Graaf W, et al. Portal vein embolization before liver resection: a systematic review. Cardiovasc Intervent Radiol. 2013;36(1):25-34. https://doi.org/10.1007/s00270-012-0440-y
Laurent C, Fernandez B, Marichez A, et al. Radiological simultaneous portohepatic vein embolization (RASPE) before major hepatectomy: a better way to optimize liver hypertrophy compared to portal vein embolization. Ann Surg. 2020;272(2):199-205. https://doi.org/10.1097/SLA.0000000000003905
Zhang J, Steib CJ. New evidence for liver venous deprivation: safety and feasibility for extended liver resections. Ann Transl Med. 2020;8(19):1259. https://doi.org/10.21037/atm-20-3057
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cassese, G., Troisi, R.I., Khayat, S. et al. Liver Venous Deprivation Versus Portal Vein Embolization Before Major Hepatectomy for Colorectal Liver Metastases: A Retrospective Comparison of Short- and Medium-Term Outcomes. J Gastrointest Surg 27, 296–305 (2023). https://doi.org/10.1007/s11605-022-05551-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-022-05551-2