Abstract
Background or Purpose
Although human umbilical cord blood-derived mesenchymal stem cell transplantation (HUCB-MSCT) resulted in a good short-term therapeutic effect on patients with decompensated liver cirrhosis (DLC), the long-term survival remained unclear. This study aimed to evaluate the impact of HUCB-MSCT on long-term outcomes in patients with DLC.
Methods
This retrospective cohort study included hospitalized patients with decompensated cirrhosis in Liuzhou Hospital of Traditional Chinese Medicine between November 2010 and February 2013. The primary outcome was overall survival (OS). The secondary outcomes were 3-year and 5-year survival rates and the occurrence rate of hepatocellular carcinoma (HCC).
Results
A total of 201 subjects were enrolled, including 36 patients who underwent HUCB-MSCT (SCT group) and 165 patients who did not (non-SCT group). After PSM (1:2), there were 36 patients in the SCT group and 72 patients in non-SCT group. The 3-year and 5-year survival rates of the two groups were 83.3% vs. 61.8% and 63.9% vs. 43.6%, and median OS time was 92.50 and 50.80 months, respectively. HUCB-MSCT treatment was found to be an independent beneficial factor for patient OS (hazard ratio = 0.47; 95% CI: 0.29–0.76; P = 0.002). There was no significant difference in the occurrence rate of HCC between the two groups (P = 0.410).
Discussion or Conclusions
HUCB-MSCT may improve long-term OS without increasing the occurrence of HCC in patients with DLC.
Trial Registration
The Chinese Clinical Trial Registry (ChiCTR2100047550).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cirrhosis is a diffuse liver disease, which is pathologically characterized by the occurrence of significant fibrosis and the formation of pseudolobules.1 Despite considerable research effort, there is a lack of acceptable treatment options for cirrhosis.2 Cirrhosis progresses slowly and is caused by several conditions including chronic alcoholism, hepatitis B virus and hepatitis C virus infection, and nonalcoholic steatohepatitis.3 When liver cirrhosis progresses to the decompensated stage, liver transplantation is the ultimate effective treatment. However, the clinical application of liver transplantation is severely limited due to the shortage of donor livers, high cost, and risks of surgical injury and immune rejection.4 New treatments are urgently needed as cirrhosis is a leading cause of morbidity and mortality worldwide.5
Basic and clinical studies in recent years have shown that stem cell therapy has the potential to become an effective alternative therapy for the treatment of liver cirrhosis.6,7,8 Mesenchymal stem cells (MSCs) are the most widely studied stem cells,9,10 both experimentally and clinically, and can be obtained from many tissues including the bone marrow, umbilical cord blood, adipose tissue, and placenta.11,12,13 The use of bone marrow derived stem cells is limited by the invasiveness of the procedure and the fact that the quality of derived stem cells was dependent upon age. Compared to bone marrow stem cells, the collection of human umbilical cord blood derived mesenchymal stem cells (HUCB-MSCs) is less invasive and carries less risk to the patient.14
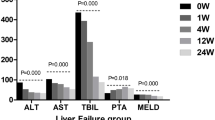
Prior studies have shown that HUCB-MSCs can safely and effectively improve liver function as shown by improvements to Child-Turcotte-Pugh (CTP) and model for end-stage liver disease (MELD) scores in patients with liver cirrhosis.15,16 However, there is a lack of data on the long-term efficacy of umbilical cord mesenchymal stem cell transplantation, particularly in patients with liver cirrhosis. Moreover, transplantation of stem cells also carries safety risks such as the potential for tumor development.17 There are currently too few data including long-term follow-up to support the long-term safety of HUCB-MSC transplantation in liver cirrhosis patients.
This study aimed to evaluate the impact of HUCB-MSCT on long-term outcomes of patients with decompensated liver cirrhosis (DLC).
Methods
Study Design and Population
This retrospective cohort study included hospitalized patients with DLC admitted to Liuzhou Hospital of Traditional Chinese Medicine (Liuzhou, China) between November 2010 and February 2013. The study was approved by the Institutional Ethics Committee of Liuzhou Hospital of Traditional Chinese Medicine. The requirement for informed consent was waived by the committee due to the nature of the retrospective study.
The inclusion criteria were: (1) diagnosis of DLC; (2) aged between 18 and 75 years. Considering that some factors may affect the objective interpretation of the data analysis, the exclusion criteria were set as follows: (1) diagnosis with another serious disease outside the liver; (2) recipient of a late stage liver transplantation; (3) diagnosis of liver cancer before treatment; and (4) incomplete data or loss to follow-up. DLC was diagnosed by previous medical history, clinical manifestation, blood and imaging examinations, or liver biopsy.
Data Collection
Clinical patient data, including age, sex, cause of disease, CTP score, CTP class, MELD score, and laboratory examination results (alanine aminotransferase (ALT), aspartate transaminase (AST), albumin (ALB), total bilirubin (TBIL), prothrombin time (PT), international normalization ratio (INR), creatinine(Cr), and platelet (PLT)), was extracted from their medical records。
All subjects were assigned into the SCT group or Non-SCT group according to whether they received HUCB-MSCT during hospitalization. Whether to accept HUCB-MSCT depends on the patient’s own will, there was no subjective artificial choice of doctors. Informed consent was signed prior to stem cell treatment. The survival outcomes and incidence of HCC were obtained by case records and confirmed by telephone calls. Follow-up was performed from the time of hospitalization to October 30, 2021, or the date of death or liver transplantation. HCC was diagnosed according to the Expert Consensus on Standardization of the Management of HCC in China. Abdominal B ultrasonography and CT or MRI scans were performed to exclude other disorders.
All patients in the study received standard clinical treatments (including albumin supplementation, coagulation correction, liver protection, antiviral treatment, and necessary anti-infective treatment) and management of complications such as ascites, variceal bleeding, infection, and hepatorenal syndromes according to updated guidelines.
Outcomes
The primary outcome was the overall survival. The secondary outcomes were 3-year and 5-year survival rates and the occurrence rates of HCC. Overall survival was defined as the time from the start of the study to the death of a case.
Statistical Analysis
Statistical analysis was conducted using the R software (version 4.0.2). Each variable was tested for normality using the Shapiro–Wilk test. Continuous variables were expressed as mean ± standard deviation (SD) if normally distributed or median (interquartile) if not normally distributed and were analyzed using a two-sample t-test or Wilcoxon rank sum test, as appropriate. Sex and etiology were expressed as the number of patients (percentage) and tested with the χ2 test or Fisher’s exact test, as appropriate. A ratio of 1 transplantation patient for every 2 non-SCT patients was achieved by propensity score matching using the MatchIt R package. Thirteen factors were included in the propensity score matching analysis including sex, age, etiology, alanine aminotransferase, aspartate aminotransferase, total bilirubin, albumin, international normalized ratio, serum sodium, creatinine, platelets, CTP score, and MELD score. Univariate and multivariate Cox proportional hazard regression analyses were performed to identify significant demographic and clinical characteristics. Survival curves were plotted using the Kaplan–Meier method and analyzed using the log-rank test. All analyses were performed as two-sided tests with a 0.05 level of significance.
Results
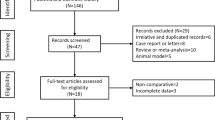
A total of 248 patients with DLC were initially enrolled, and 47 patients were excluded. Finally, a total of 201 subjects were enrolled, including 36 patients undergoing HUCB-MSCT and 165 patients without transplantation. Except for albumin (ALB) levels, there were no significant differences between the non-SCT group and the SCT group (all P > 0.05) before PSM. The bias was addressed by limiting the ratio of cases to non-SCTs to 1 to 2, which resulted in 36 patients in SCT group and 72 patients in non-SCT group with comparable baseline characteristics (Table 1).
Before PSM, the overall mortality was 80.1% (161/201) in all study subjects, with 61.1% (22/36) mortality in the SCT group and 84.2% (139/165) in the Non-SCT group (P = 0.002). The median OS for the SCT group was 92.50 (95% CI, 57.5–NA) months, and 50.80 (95%CI, 39.8–64.5) months for the non-SCT group. After PSM, the overall mortality was 74.1% (80/108) in study subjects, with 61.1% (22/36) mortality in the SCT group and 80.6% (58/72) in the non-SCT group (P = 0.030). The median OS for SCT group was 92.50 (95%CI, 57.5–NA) months, and for Non-SCT group was 57.7 (95%CI, 38.0–74.2) months. The 3-year and 5-year survival rates between the two groups were 83.3% vs. 61.8% and 63.9% vs. 43.6%, respectively. After PSM, the 3-year and 5-year survival rates between the two groups were 83.3% vs. 63.9% and 63.9% vs. 47.2%, respectively. The Kaplan–Meier survival curves before and after PSM were significantly higher in the SCT group than in the non-SCT group (all P < 0.05) (Fig. 1). By multivariate regression analysis, HUCB-MSCT treatment was found to be an independent benefificial factor for patient OS (Table 2).
In the entire cohort, the overall incidence of HCC was 33.3% (67/201), with 41.7% (15/36) incidence in the SCT group and 31.5% (52/165) in the non-SCT group (P = 0.242). In the matched cohort, the overall incidence of HCC was 32.4% (35/108), with 41.7% (15/36) incidence in the SCT group and 27.8% (20/72) in the non-SCT group (P = 0.146). No significant difference was found in the cumulative risk function of HCC between the two groups before and after PSM (all P > 0.05) (Fig. 2).
Discussion
This study demonstrates that HUCB-MSCT can improve survival outcomes in patients with liver cirrhosis. Additionally, the results found that HUCB-MSCT did not increase the risk of HCC in these patients. We therefore conclude that HUCB-MSCT may be a safe and effective method for the treatment of liver cirrhosis, both in the long- and short-term.
A large-scale meta-analysis of randomized non-SCT led trials (RCTs) evaluating the therapeutic effects and safety of stem cell therapy for chronic liver disease (CLD) revealed that stem cell therapy is a safe and effective therapeutic option for CLD.18 HUCB-MSCTs have shown great potential in regenerative medicine due to their multilineage differentiation potential, low immunogenicity, and self-renewal ability.19,20 Many recent studies have shown that patients with decompensated cirrhosis receive significant short-term benefits from HUCB-MSCT.18,19,20 However, there was a lack of data demonstrating the long-term benefit of HUCB-MSCT in patients with decompensated cirrhosis. In addition, most previous clinical studies only evaluated the safety of this procedure in the short-term. In view of the possible tumorigenicity of stem cell therapy, long-term follow-up observation was necessary.
In contrast with this report, a recent meta-analysis which examined clinical outcomes of stem cell transplantation from various human tissue sources in cirrhotic patients showed no significant difference in the mortality between the treatment and non-SCT groups, and concluded that HUCB-MSCT could improve liver function but appeared to not be significant in increasing the survival in cirrhotic patients.21 However, in another clinical study, autologous transplants of peripheral blood stem cells significantly improved long-term survival as compared to the non-SCT group.22 This difference in outcome may be related to differing source of cells, methods of transplantation, and degrees of liver cirrhosis.
Patients with cirrhosis are at increased risk of developing HCC. It was an open question if stem cell transplantation could potentially further increase the incidence of HCC in patients with cirrhosis. A recent study recommended close monitoring of patients undergoing autologous SCT for HCC.23 However, this conflicts with another report and our study which did not find a difference in HCC prevalence between standard medical treatment and peripheral blood stem cells treatment.22 In a high carcinogenic risk liver cirrhosis mouse model, no significant differences were observed between the non-SCT and bone marrow stem cell treatment groups in the incidence, number, or mean size of lesions and tumors on histological evaluation.24 Our long-term follow-up results showed that HUCB-MSCT did not increase the risk of HCC. Moreover, research has shown that stem cell may improve the immunologic status and microenvironment of target tissues and therefore potentially may delay HCC development.25 However, further studies are necessary to fully understand the effects of stem cell treatment on HCC development.
However, there are several limitations of this study. Firstly, it was a retrospective study and selection biases may have existed since this study was subject to the inherent limitations associated with retrospective analyses. Secondly, as a single-center study, the sample size of this study was not large enough to draw firm conclusions. In the future, large-scale and prospective studies are required to clarify the long-term survival benefit and safety of HUCB-MSCTs in patients with decompensated cirrhosis.
In conclusion, this study suggests HUCB-MSCT may improve long-term survival without increasing the risks of HCC in patients with decompensated cirrhosis.
Data Availability
All data generated or analysed during this study are included in this published article.
Abbreviations
- HBV:
-
Hepatitis B virus
- ALT:
-
Alanine aminotransferase
- UCMSCs:
-
Umbilical cord mesenchymal stem cells
- ACLF:
-
Acute-on-chronic liver failure
- TBIL:
-
Total bilirubin
- PTA:
-
Prothrombin activity
- MELD:
-
Model for end-stage liver disease
References
Ginès P, Krag A, Abraldes JG, Solà E, Fabrellas N, Kamath PS. Liver cirrhosis. Lancet 2021;398:1359-1376.
Campana L, Iredale JP. Regression of Liver Fibrosis. Semin Liver Dis 2017;37:1-10.
Ge PS, Runyon BA. Treatment of Patients with Cirrhosis. N Engl J Med 2016;375:767-777.
Belli LS, Duvoux C, Artzner T, Bernal W, Conti S, Cortesi PA, Sacleux SC, Pageaux GP, Radenne S, Trebicka J, Fernandez J, Perricone G, Piano S, Nadalin S, Morelli MC, Martini S, Polak WG, Zieniewicz K, Toso C, Berenguer M, Iegri C, Invernizzi F, Volpes R, Karam V, Adam R, Faitot F, Rabinovich L, Saliba F, Meunier L, Lesurtel M, Uschner FE, Fondevila C, Michard B, Coilly A, Meszaros M, Poinsot D, Schnitzbauer A, De Carlis LG, Fumagalli R, Angeli P, Arroyo V, Jalan R. Liver transplantation for patients with acute-on-chronic liver failure (ACLF) in Europe: Results of the ELITA/EF-CLIF collaborative study (ECLIS). J Hepatol 2021;75:610-622.
Moon AM, Singal AG, Tapper EB. Contemporary Epidemiology of Chronic Liver Disease and Cirrhosis. Clin Gastroenterol Hepatol 2020;18:2650-2666.
de Miguel MP, Prieto I, Moratilla A, Arias J, Aller MA. Mesenchymal Stem Cells for Liver Regeneration in Liver Failure: From Experimental Models to Clinical Trials. Stem Cells Int 2019;2019:3945672.
Wu CX, Wang D, Cai Y, Luo AR, Sun H. Effect of Autologous Bone Marrow Stem Cell Therapy in Patients with Liver Cirrhosis: A Meta-analysis. J Clin Transl Hepatol 2019;7:238-248.
Sun A, Gao W, Xiao T. Autologous bone marrow stem cell transplantation via the hepatic artery for the treatment of hepatitis B virus-related cirrhosis: a PRISMA-compliant meta-analysis based on the Chinese population. Stem Cell Res Ther 2020;11:104.
Mizukami A, Swiech K. Mesenchymal Stromal Cells: From Discovery to Manufacturing and Commercialization. Stem Cells Int 2018;2018:4083921.
Yamada Y, Nakamura-Yamada S, Kusano K, Baba S. Clinical Potential and Current Progress of Dental Pulp Stem Cells for Various Systemic Diseases in Regenerative Medicine: A Concise Review. Int J Mol Sci 2019;20.
Yu YB, Song Y, Chen Y, Zhang F, Qi FZ. Differentiation of umbilical cord mesenchymal stem cells into hepatocytes in comparison with bone marrow mesenchymal stem cells. Mol Med Rep 2018;18:2009-2016.
Suk KT, Yoon JH, Kim MY, Kim CW, Kim JK, Park H, Hwang SG, Kim DJ, Lee BS, Lee SH, Kim HS, Jang JY, Lee CH, Kim BS, Jang YO, Cho MY, Jung ES, Kim YM, Bae SH, Baik SK. Transplantation with autologous bone marrow-derived mesenchymal stem cells for alcoholic cirrhosis: Phase 2 trial. Hepatology 2016;64:2185-2197.
Hu C, Zhao L, Li L. Current understanding of adipose-derived mesenchymal stem cell-based therapies in liver diseases. Stem Cell Res Ther 2019;10:199.
Song JS, Hong KT, Kim NM, Park HS, Choi NH. Human umbilical cord blood-derived mesenchymal stem cell implantation for osteoarthritis of the knee. Arch Orthop Trauma Surg 2020;140:503-509.
El Baz H, Demerdash Z, Kamel M, Atta S, Salah F, Hassan S, Hammam O, Khalil H, Bayoumi A. Potentials of Differentiated Human Cord Blood-Derived Unrestricted Somatic Stem Cells in Treatment of Liver Cirrhosis. Exp Clin Transplant 2019;17:251-258.
Tao H, Li Y, Wang T, Zhou C. Umbilical cord blood stem cells transplantation as an adjunctive treatment strategy for liver cirrhosis in Chinese population: a meta-analysis of effectiveness and safety. Ther Clin Risk Manag 2018;14:417-440.
Lezmi E, Benvenisty N. The Tumorigenic Potential of Human Pluripotent Stem Cells. Stem Cells Transl Med 2022.
Zhou GP, Jiang YZ, Sun LY, Zhu ZJ. Therapeutic effect and safety of stem cell therapy for chronic liver disease: a systematic review and meta-analysis of randomized controlled trials. Stem Cell Res Ther 2020;11:419.
Qiao Y, Xu Z, Yu Y, Hou S, Geng J, Xiao T, Liang Y, Dong Q, Mei Y, Wang B, Qiao H, Dai J, Suo G. Single cell derived spheres of umbilical cord mesenchymal stem cells enhance cell stemness properties, survival ability and therapeutic potential on liver failure. Biomaterials 2020;227:119573.
Kim JY, Jeon HB, Yang YS, Oh W, Chang JW. Application of human umbilical cord blood-derived mesenchymal stem cells in disease models. World J Stem Cells 2010;2:34-38.
Qi X, Guo X, Su C. Clinical outcomes of the transplantation of stem cells from various human tissue sources in the management of liver cirrhosis: a systematic review and meta-analysis. Curr Stem Cell Res Ther 2015;10:166-180.
Guo C, Guo G, Zhou X, Chen Y, Han Z, Yang C, Zhao S, Su H, Lian Z, Leung PSC, Gershwin ME, Zhou X, Han Y. Long-term Outcomes of Autologous Peripheral Blood Stem Cell Transplantation in Patients With Cirrhosis. Clin Gastroenterol Hepatol 2019;17:1175-1182.e1172.
Wang MF, Li YB, Gao XJ, Zhang HY, Lin S, Zhu YY. Efficacy and safety of autologous stem cell transplantation for decompensated liver cirrhosis: A retrospective cohort study. World J Stem Cells 2018;10:138-145.
Matsuura K, Takami T, Maeda M, Hisanaga T, Fujisawa K, Saeki I, Matsumoto T, Hidaka I, Yamamoto N, Sakaida I. Evaluation of the Effects of Cultured Bone Marrow Mesenchymal Stem Cell Infusion on Hepatocarcinogenesis in Hepatocarcinogenic Mice With Liver Cirrhosis. Transplant Proc 2019;51:925-935.
Cui L, Shi Y, Han Y, Fan D. Immunological basis of stem cell therapy in liver diseases. Expert Rev Clin Immunol 2014;10:1185-1196.
Funding
This study is supported by the National Natural Science Foundation of China (No. 81500462 and No. 82160882).
Author information
Authors and Affiliations
Contributions
ZPL, XLC, XLZ, MJS, and HMX contributed to the study design, data analysis and interpretation, manuscript writing, and manuscript revision. ZPL, LH, and ML contributed to the data collection and processing. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics Approval
This is a retrospective cohort study. This procedure used in this study adhered to the tenets of the Declaration of Helsinki, and informed consent was obtained from all patients. The study was approved by the Institutional Ethics Committee of Liuzhou Hospital of Traditional Chinese Medicine.
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Li, Z., Zhou, X., Han, L. et al. Human Umbilical Cord Blood-Derived Mesenchymal Stem Cell Transplantation for Patients with Decompensated Liver Cirrhosis. J Gastrointest Surg 27, 926–931 (2023). https://doi.org/10.1007/s11605-022-05528-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-022-05528-1