Abstract
Background
Bile leakage (BL) is one of the commonest morbidities after hepatic resection for hepatocellular carcinoma (HCC). The current study was conducted to evaluate the incidence and different predictive factors for BL after hepatic resection for HCC, and to evaluate of the impact of BL on the long-term survival outcomes.
Methods
We reviewed the patients’ data who underwent hepatic resection for HCC during the period between June 2010 and June 2019.
Results
A total of 293 patients were included in the study. BL occurred in 17 patients (5.8%). More Child–Pugh class B patients were found in BL group. There were no significant differences between the two groups except for tumor site, macroscopic portal vein invasion, extent of liver resection, Pringle maneuver use, intraoperative blood loss, and transfusions. Longer hospital stay, higher grades of post-hepatectomy liver failure, and abdominal collections were noted in BL group. After median follow-up duration of 17 months (4–110 months), there were no significant differences between BL and non-BL group regarding overall survival (log-rank, p = 0.746) and disease-free survival (log-rank, p = 0.348). In multivariate analysis, Child–Pugh class, macroscopic portal vein invasion, liver resection extent (minor/major), and Pringle’s maneuver use were the only significant predictors of BL.
Conclusion
BL did not significantly impair the long-term outcomes after hepatic resection for HCC. Child–Pugh class, macroscopic portal vein invasion, liver resection extent (minor/major), and Pringle’s maneuver use were the main risk factors of BL in the current study.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hepatic resection plays an essential role in the curative treatment of hepatocellular carcinoma (HCC) patients. With improvement of the patients’ selection criteria, refinement of surgical techniques, and advancement of perioperative care, the outcomes of hepatic resection have markedly improved in the recent years .1,2 The most important complications after hepatic resection for HCC include postoperative hemorrhage, post-hepatectomy liver failure, bile leakage (BL), and intra-abdominal infections.3
In spite of the overall decrease in the incidence of perioperative morbidities after hepatic resection, BL remains one of the most common morbidities after hepatic resection for HCC. The incidence of BL after hepatic resection remains controversial between the different studies, ranging between 2.6 and 12%.3,4,5 This attributed to the differences in the underlying hepatic parenchymal background, liver resection extent, techniques in hepatic parenchymal transection, and different modalities used for biliostasis on transection plane like hemostatic agents and fibrin glue.
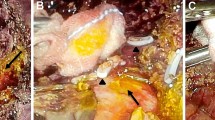
BL is one of the most feared morbidities after hepatic resection for HCC. BL is associated with increased the risk for intra-abdominal collections, sepsis, and liver decompensation. Also, it may predispose to early postoperative mortality.2 On the other hand, the impact of biliary leakage on the long-term outcomes of hepatic resection for HCC is not well elucidated.
The current study was conducted to evaluate the incidence and different predictive factors for the development of BL after hepatic resection for HCC, defined according to the International Study Group of Liver Surgery (ISGLS), in area where hepatitis C virus (genotype 4) is the main predisposing factor for HCC development 6; and, also, to evaluate of the impact of BL on the survival outcomes after hepatic resection for HCC.
Materials and Methods
Study Design
We retrospectively reviewed the data of patients who underwent primary liver resection for pathologically confirmed HCC at Gastro-Intestinal Surgery Center, Mansoura University, Egypt, during the period between June 2010 and June 2019. Patient data were retrieved from a prospectively maintained database for all patients undergoing liver resection. An informed consent was obtained from each patient prior to surgical intervention. The study was approved by the Institutional Review Board and Local Ethical Committee at the Faculty of Medicine, Mansoura University, Egypt (Code Number: R.20.06.875).
Preoperative Evaluation
Preoperative workup included detailed laboratory and radiological evaluation, as previously shown.7,8 Selection of appropriate treatment strategy was discussed at multidisciplinary meetings. Generally, liver resection was applied for patients with preserved liver functions, without signs of severe portal hypertension, without evidence of extrahepatic metastasis, and with American Society of Anesthesiologists (ASA) grade < III.9
Surgical Procedure
The surgical procedure had been described previously.7,8,10 Generally, parenchymal sparing liver resection was preferred. Major liver resections were performed for patients with large tumors or tumors close to major hepatic vasculature if the future remnant liver is adequate (more than 40% of the total liver volume). Volumetric assessment was performed for selected patients requiring major liver resection with marginal liver functions. Otherwise, non-anatomical liver resections were more preferred.
Parenchymatous transection was performed by combinations of clamp-crush method and ultrasonic devices. Intermittent Pringle’s maneuver was applied selectively during liver transection. Intraoperative ultrasonography was utilized in some patients to check the resection margin and exclude presence of multifocal tumors. Intraoperative cholangiography was performed in some patients to ensure biliostasis and assess the remnant biliary system integrity.
Postoperative Care and Follow-up
After surgery, patients were transferred to the intensive care unit or to the ward for monitoring of vital signs and routinely inserted abdominal drains. All patients underwent daily laboratory evaluation. Abdominal ultrasonography was performed routinely in all patients. Oral fluids were started once intestinal sounds were restored. Abdominal drains were removed when daily output was less than 100 cc with absence of any abdominal collections. After discharge, patients were followed up in the outpatient clinic. Follow-up visit included routine laboratory and radiological evaluation.
Study Outcomes
The primary outcome of the study is the incidence of BL, defined according to the International Study Group of Liver Surgery (ISGLS).6 Secondary outcomes included evaluation of the impact of BL on overall survival (OS) and disease-free survival (DFS), also to evaluate different predictive factors for the development of BL after liver transection for HCC.
Definitions
The types of liver resection were defined according to Brisbane 2000 terminology.11 Liver resections were classified into minor (≤ 2 segments) or major (≥ 3 segments) according to Couinaud classification. Postoperative morbidity is defined as adverse events happening during the early postoperative period and is graded according to the Clavien-Dindo classification.12 Postoperative BL was defined according to the International Study Group of Liver Surgery (ISGLS).6 Post-hepatectomy liver failure was defined according to the ISGLS definition.13
Early postoperative mortality was defined as mortality occurring during the first 90 postoperative days, and was excluded from further survival analysis. OS was calculated from the day of surgery to the day of confirmed death or the last follow-up visit. DFS was calculated from the day of surgery to the day of confirmed tumor recurrence or the day of death or last follow-up.
Statistical Analysis
Shapiro–Wilk test is used to assess the normality of continuous data. Categorical variables are expressed as number and percentage, and continuous variables are expressed as median and range. Comparison between groups is done by chi-square test for categorical variables and Mann–Whitney test for continuous variables. Survival analysis is performed by Kaplan–Meier method and comparison between groups is done by log-rank test.
Univariate and multivariate analyses are done by binary logistic regression analysis to identify the independent risk factors for BL. Significant factors determined in the univariate analysis are included in the subsequent multivariate analysis. Statistical analysis of the data is performed using IBM-SPSS software for Windows, version 24 (IBM Corp., Armonk, NY). A p value < 0.05 is considered significant.
Results
During the period between June 2010 and June 2019, 293 patients underwent liver resection for HCC at Gastro-Intestinal Surgery Center, Mansoura University, Egypt. Patients were divided into two groups according to the occurrence of BL, non-bile leakage (non-BL) group (276 patients 94.2%), and bile leakage (BL) group (17 patients 5.8%).
Demographic Data
Demographic data of the study patients are shown in Table 1. There were no significant differences between the two groups except for Child–Pugh score. More Child–Pugh class A patients were found in non-BL group while more Child–Pugh class B patients were found in BL group. Hepatitis C virus infection was the most common underlying cause for HCC among the study patients.
Radiological and Endoscopic Data
Radiological and endoscopic data of the study patients are summarized in Table 2. There were no significant differences between the groups regarding preoperative radiological and endoscopic data apart from presence of macroscopic portal vein invasion. Macroscopic portal vein invasion was more observed in BL group (5 patients 29.4%) compared to non-BL groups (31 patients 11.2%) (p = 0.043).
Operative Data
Operative data of the study patients are summarized in Table 3. There were significant differences between the two groups regarding tumor site, macroscopic portal vein invasion, extent of liver resection, type of liver resection, Pringle maneuver use, intraoperative blood loss, and blood transfusions.
Postoperative Data
Postoperative data of the study patients are summarized in Table 4. Longer hospital stay was noted in BL group (15 vs 5 days, p = 0.001). Higher grades of post-hepatectomy liver failure (grade B and C) and postoperative abdominal collections were noted in BL group.
Pathological Outcomes
Pathological data of the study patients are summarized in Table 5. There were no significant differences between the two groups regarding different pathological data.
Survival Outcomes
Overall Survival
The median follow-up duration was 17 months (4–110 months). Mortality occurred in 89 patients (30.4%). The 1-, 3-, and 5-year OS rates for all study patients were 85.9%, 68.6%, and 49.5%, respectively (Fig. 1A). The 1-, 3-, and 5-year OS rates for the non-BL group were 86.2%, 67.8%, and 49.3%, respectively. The 1-, 3-, and 5-year OS rates for BL group were 79.6%, 70.8%, and 70.8%, respectively (log-rank, p = 0.746) (Fig. 2A).
Disease-Free Survival
Recurrence occurred in 133 patients (45.4%). There were no significant differences between the groups regarding recurrence time, and recurrence management as shown in Table 6. More extrahepatic recurrences occurred in BL group (p = 0.017). The 1-, 3-, and 5-year DFS rates for all study patients were 74.3%, 42.8%, and 26.7%, respectively (Fig. 1B). The 1-, 3-, and 5-year DFS rates for the non-BL group were 73.2%, 42.2%, and 26.2%, respectively. The 1-, 3-, and 5-year DFS rates for BL group were 84.4%, 50.6%, and 50.6%, respectively (log-rank, p = 0.348) (Fig. 2B).
Predictive Factors for Bile Leakage
Predictive factors for BL are shown in Table 7. In univariate analysis, Child–Pugh class, tumor site, macroscopic portal vein invasion, liver resection extent (minor/major), Pringle’s maneuver, and operation time were significantly correlated with BL. In multivariate analysis, Child–Pugh class, macroscopic portal vein invasion, liver resection extent (minor/major), and Pringle’s maneuver were the only significant predictors of BL.
Discussion
With the recent advancements in the surgical techniques and perioperative patients’ care, the rate of perioperative mortality after hepatic resection for HCC has dramatically improved.14 However, the rates of perioperative morbidities remain a major concern. The most important complications after hepatic resection for HCC include postoperative hemorrhage, post-hepatectomy liver failure, BL, and intra-abdominal infections.3 BL after hepatic resection for HCC continue to be a common reason of major perioperative morbidity.15,16
Previous studies had shown variable incidence of BL following hepatic resection for various benign and malignant liver tumors ranging between 2.6 and 12%.3,4,5,15,16,17 The great differences in the BL incidence between the different studies are related to lack of standardized definition of BL after hepatic resection. The International Study Group of Liver Surgery (ISGLS) has proposed a new consensus definition of BL following hepato-biliary surgeries based on the postoperative course of bilirubin concentrations in the serum and the abdominal drainage fluid.6 The definition is simple, easy to apply, and enabled comparison of the results between the different clinical studies. Also, it enabled objective assessment of various treatment modalities.6 In the current study, BL occurred in 17 patients (5.8%). Some studies had shown a close relationship between BL and high postoperative mortality.3,18,19 This is attributed to high risk of septic complications associated with BL which may progress to liver failure and ascites. In the current study, we experienced higher grades of post-hepatectomy liver dysfunction and abdominal collections. However, they were carefully managed, and we did not experience any postoperative mortality due to BL. This is attributed to close monitoring and early intervention for cases with BL among our series. Six cases (35.3%) were managed conservatively, three cases (17.6%) required radiologic intervention, seven cases (41.2%) required endoscopic intervention, and only one case (5.9%) required surgical intervention. In the current study, we noticed high incidence of post-hepatectomy liver failure among our patients.8 We also noticed higher incidence of post-hepatectomy liver failure in patients with BL; however, this was not statistically significant. The high incidence of post-hepatectomy liver failure in BL group was more related to underlying liver parenchymal dysfunction and the extent of liver resection rather than BL.
Several previous studies analyzed different risk factors for BL after various types of hepatic resection. These risk factors include preoperative ablation, liver cirrhosis, mixed hepatocellular-cholangiocarcinoma, prolonged operation time, extended liver resections, resections involving segment IV, and repeated hepatic resection.16,20,21 In the current study, we found that preoperative Child–Pugh class, macroscopic portal vein invasion, liver resection extent (minor/major), and Pringle’s maneuver use were the only significant predictors of BL in multivariate analysis.
HCC usually develops on a background of liver cirrhosis. A previous study by Tanaka et al. reported that patients with liver cirrhosis experienced lower incidence BL but the difference was not statistically significant.22 Capussotti et al. found that patients with liver cirrhosis experienced a lower rate of BL during the postoperative course after hepatic resection.3 This is attributed to the extent of liver resection that is applied in patients with liver cirrhosis. Minor resections are usually applied in this group of patients, with a lower rate of extended and major resections. In the current study, we found that the degree of severity of liver cirrhosis as demonstrated by Child–Pugh score was a significant predictor for BL after hepatic resection for HCC. We also found higher incidence of child B cirrhosis in patients who experienced biliary leakage. Also, these groups of patients underwent more major hepatic resections compared to patients who did not experience biliary leakage. It has been reported that biliary leakage is more common after extended and major hepatic resections.2 In major hepatic resections, extended transection planes are usually utilized that is often extended out of the portal fissures and with an extensive dissection of the hepatic duct close to the hilar confluence.23
Several previous studies have stated that partial hepatic resection in which there is exposure of major Glissonian pedicles during parenchymatous transection such as central bisectionectomy, segment IV resection, and segment VIII resection were independent predictors of BL.3,17,18,21 In the current study, we found that extended liver resections (major liver resections) were significantly associated with the risk for the development of biliary leakage. However, we did not find any significant correlation of different types of liver resection and biliary leakage. Tumors requiring major and extended liver resections require transection planes that is often extended out of the portal fissures and with extensive dissection of the hepatic duct close to the hilar confluence to achieve tumor-free margins.
Also, we found that a prolonged operating time and pringle maneuver use were independent risk factors for BL after hepatic resection on univariate analysis and only pringle maneuver use on multivariate analysis. A prolonged operation time and pringle maneuver use are potential indicators of hepatic resections that are technically difficult or require complicated transection planes.
Finally, we analyzed the impact of BL on the long-term survival outcomes of liver resection for HCC. We hypothesized that patients who experienced biliary leakage will have worse long-term survival outcomes. After a median follow-up duration of 17 months (4–110 months), patients with biliary leakage experienced relatively lower overall and disease-free survival outcomes; however, this was not statistically significant. Few studies had evaluated the impact of BL on the long-term outcomes after hepatic resection especially for HCC. Concerns have been raised regarding the progression of different liver tumors after major biliary leakage following hepatic resections. Braunwarth et al. analyzed the impact of BL on the survival outcomes after hepatic resection of different liver tumors. They found a relevant influence of biliary leakage on OS in perihilar cholangiocarcinoma, whereas no association was seen in other liver tumors, indicating that tumor progression might be triggered by BL in cancer types arising from the bile ducts itself.24 Yamamoto et al. in a study evaluating the long-term impact of BL after liver resection for HCC found that post-operative BL was strongly associated with poorer OS and DFS following liver resection and may contribute to our understanding of the requirements for preventing BL and developing strategies towards achieving improved treatment outcomes of postoperative BL.25
The current study has several limitations including its retrospective nature; it included data from a single center, and it included limited number of patients. Also, the number of bile leakage events was relatively small. A future multicenter study including large number of cases from our locality is required to support our findings.
Conclusion
BL is one of the commonest morbidities after hepatic resection for HCC. It is associated with prolonged hospital stay, higher grades of post-hepatectomy liver failure, and frequent abdominal collections. However, it is not associated with higher perioperative mortality when carefully managed. Child–Pugh class, macroscopic portal vein invasion, liver resection extent (minor/major), and Pringle’s maneuver use were the main risk factors of BL in this series of patients. BL did not significantly impair the long-term outcomes of this group of patients.
References
Reed DN Jr, Vitale GC, Wrightson WR, Edwards M, McMasters K: Decreasing mortality of bile leaks after elective hepatic surgery. Am J Surg 2003; 185: 316–318.
Erdogan D, Busch OR, Gouma DJ, van Gulik TM. Prevention of biliary leakage after partial liver resection using topical hemostatic agents. Digestive Surgery. 2007;24(4):294-9.
Capussotti L, Ferrero A, Viganò L, Sgotto E, Muratore A, Polastri R. Bile leakage and liver resection: where is the risk? Archives of surgery. 2006 Jul 1;141(7):690-4.
Imamura H, Seyama Y, Kokudo N, et al. One thousand fifty-six hepatectomies without mortality in 8 years. Arch Surg. 2003;138:1198-1206.
Jarnagin WR, Gonen M, Fong Y, et al. Improvement in perioperative outcome after hepatic resection: analysis of 1,803 consecutive cases over the past decade. Ann Surg. 2002;236:397-407)
Koch M, Garden OJ, Padbury R, Rahbari NN, Adam R, Capussotti L, Fan ST, Yokoyama Y, Crawford M, Makuuchi M, Christophi C. Bile leakage after hepatobiliary and pancreatic surgery: a definition and grading of severity by the International Study Group of Liver Surgery. Surgery. 2011 May 1;149(5):680-8.
Shehta A, Farouk A, Elghawalby AN, Elshobary M, Aboelenin A, Fouad A, Ali MA. Outcomes of hepatic resection for hepatocellular carcinoma associated with portal vein invasion. Journal of Surgical Research. 2021 Oct 1;266:269-83.
Shehta A, Farouk A, Fouad A, Aboelenin A, Elghawalby AN, Said R, Elshobary M, El Nakeeb A. Post-hepatectomy liver failure after hepatic resection for hepatocellular carcinoma: a single center experience. Langenbeck’s Archives of Surgery. 2021 Feb;406(1):87-98.
Wolters U, Wolf T, Stutzer H, Schroder T (1996) ASA classification and perioperative variables as predictors of postoperative outcomes. Br J Anaesth 77:217–222.
Wahab MA, Shehta A, Hamed H, El Nakeeb A, Salah T (2014) Predictors of recurrence in hepatitis C virus related hepatocellular carcinoma after hepatic resection: a retrospective cohort study. The Eurasian journal of medicine 46(1):36-41
Terminology Committee of the International Hepato-Pancreato- Biliary Association (2000) The IHPBA Brisbane 2000 terminology of liver anatomy and resections. HPB Surg 2:333-339.
Dindo D, Demartines N, Clavien PA (2004) Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240:205-213.
Rahbari NN, Garden OJ, Padbury R, et al (2011) Posthepatectomy liver failure: a definition and grading by the International Study Group of Liver Surgery (ISGLS). Surgery 149:713-724.
Torzilli G, Makuuchi M, Inoue K, Takayama T, Sakamoto Y, Sugawara Y et al. No-mortality liver resection for hepatocellular carcinoma in cirrhotic and noncirrhotic patients: is there a way? A prospective analysis of our approach. Arch Surg 2007; 134: 984–992.
Virani S, Michaelson J, Hutter M, Lancaster RT, Warshaw AL, HendersonWG et al. Morbidity and mortality after liver resection: results of the patient safety in surgery study. J Am Coll Surg 2007; 204: 1284–1292.
Sadamori H, Yagi T, Matsuda H, Shinoura S, Umeda Y, Yoshida R et al. Risk factors for major morbidity after hepatectomy for hepatocellular carcinoma in 293 recent cases. J Hepatobiliary Pancreat Sci 2010; 17: 709–718.
Yamashita Y, Hamatsu T, Rikimaru T, Tanaka S, Shirabe K, Shimada M et al. Bile leakage after hepatic resection. Ann Surg 2001; 233: 45–50.
Zheng SM, Li H, Li GC, Yu DS, Ying DJ, Zhang B, Lu CD, Zhou XH. Risk factors, treatment and impact on outcomes of bile leakage after hemihepatectomy. ANZ Journal of Surgery. 2017 Jul;87(7-8):E26-31.
Lo CM, Fan ST, Liu CL, et al. Biliary complications after hepatic resection: risk factors, management, and outcome. Arch Surg. 1998;133:156-161.
Okumura K, Sugimachi K, Kinjo N, Shoji F, Ikebe M, Makino I, Higashi H. Risk factors of bile leakage after hepatectomy for hepatocellular carcinoma. Hepato-gastroenterology. 2013;60(127):1717-9.
Nagano Y, Togo S, Tanaka K, Masui H, Endo I, Sekido H et al. Risk factors and management of bile leakage after hepatic resection. World J Surg 2003; 27: 695–698.
Tanaka S, Hirohashi K, Tanaka H, et al. Incidence and management of bile leakage after hepatic resection for malignant hepatic tumors. J Am Coll Surg. 2002;195:484-489.
Uenishi T, Hirohashi K, Kubo S, et al. Clinicopathological factors predicting outcome after resection of mass-forming intrahepatic cholangiocarcinoma. Br J Surg. 2001;88:969-974.
Braunwarth E, Primavesi F, Göbel G, Cardini B, Oberhuber R, Margreiter C, Maglione M, Schneeberger S, Öfner D, Stättner S. Is bile leakage after hepatic resection associated with impaired long-term survival?. European Journal of Surgical Oncology. 2019 Jun 1;45(6):1077-83.
Yamamoto M, Kobayashi T, Kuroda S, Hamaoka M, Honmyo N, Yamaguchi M, Takei D, Ohdan H. Impact of postoperative bile leakage on long‐term outcome in patients following liver resection for hepatocellular carcinoma. Journal of Hepato‐Biliary‐Pancreatic Sciences. 2020 Dec;27(12):931-41.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
Conception and design of the manuscript: Ahmed Shehta, Ahmed Farouk.
Acquisition of data: Ahmed Farouk, Ahmed Shehta.
Analysis and interpretation of data: Ahmed Shehta, Ahmed Farouk, Ayman Elnakeeb.
Drafting the manuscript: all authors.
Critical revision of the final version to be published: all authors.
Approval of the final version to be published: all authors.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Shehta, A., Farouk, A., Said, R. et al. Bile Leakage After Hepatic Resection for Hepatocellular Carcinoma: Does It Impact the Short- and Long-term Outcomes?. J Gastrointest Surg 26, 2070–2081 (2022). https://doi.org/10.1007/s11605-022-05433-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-022-05433-7