Abstract
Objectives
To identify predictors, patterns, and timing of recurrence after resection of invasive carcinomas arising in association with an IPMN.
Background
Postoperative management of an invasive carcinoma arising in association with an intraductal papillary mucinous neoplasm (IPMN), a biologically distinct entity from PanIN-derived pancreatic ductal adenocarcinoma (PDAC), remains largely based on guidelines for PanIN-derived PDAC. To minimize treatment failure and inform disease-specific management, cancer recurrence must be better characterized.
Methods
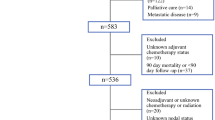
Patients were identified from a prospectively maintained registry between 1996 and 2018. Predictors of recurrence were evaluated by employing Cox regression models to determine risk-adjusted hazard ratios (HR) with 95% confidence intervals (95%CI). The patterns and timing of recurrence were recognized and compared utilizing a log-rank test, respectively.
Results
Of the 213 patients included, 92 (43.2%) recurred with a median RFS of 23.7 months (16.7–30.7). The predominant pattern of recurrence included any systemic (65.2%). The median time to local recurrence was longer than systemic (21.6 versus 11.4 months, p = 0.05). Poor differentiation [HR: 3.01, 95%CI (1.06–8.61)] and nodal disease [N1, HR: 2.23, 95%CI (1.12–4.60); and N2, HR: 5.67 95%CI (2.93–10.99)] emerged as independent predictors of systemic recurrence. For local-specific recurrences, poor differentiation [HR: 3.73, 95%CI (1.04–13.45)] and an R1 margin [high-grade dysplasia or invasive carcinoma; HR: 2.66, 95%CI (1.14–6.21)] emerged as independent predictors.
Conclusions
The predominant pattern of recurrence after resection of invasive carcinomas arising in association with IPMNs is systemic, and occurs earlier than local recurrence. Poor differentiation and nodal disease are associated with systemic recurrence while poor differentiation and an R1 margin are associated with local recurrence. Future studies should investigate the role of systemic (chemotherapy) versus local (radiation) therapies and surveillance strategies in a personalized manner.



Similar content being viewed by others
References
Kromrey ML, Bülow R, Hübner J, et al. Prospective study on the incidence, prevalence and 5-year pancreatic-related mortality of pancreatic cysts in a population-based study. Gut. 2018;67(1):138-145.
van Huijgevoort NCM, Del Chiaro M, Wolfgang CL, van Hooft JE, Besselink MG. Diagnosis and management of pancreatic cystic neoplasms: current evidence and guidelines. Nat Rev Gastroenterol Hepatol. 2019;16(11):676-689.
Adsay V, Mino-Kenudson M, Furukawa T, et al. Pathologic Evaluation and Reporting of Intraductal Papillary Mucinous Neoplasms of the Pancreas and Other Tumoral Intraepithelial Neoplasms of Pancreatobiliary Tract: Recommendations of Verona Consensus Meeting. Ann Surg. 2016;263(1):162-177. https://doi.org/10.1097/SLA.0000000000001173
Tanaka M, Fernández-del Castillo C, Adsay V, et al. International consensus guidelines 2012 for the management of IPMN and MCN of the pancreas. Pancreatology. 2012;12(3):183-197. https://doi.org/10.1016/j.pan.2012.04.004
[5] Tanaka M, Fernández-Del Castillo C, Kamisawa T, et al. Revisions of international consensus Fukuoka guidelines for the management of IPMN of the pancreas. Pancreatology. 2017;17(5):738-753. doi:https://doi.org/10.1016/j.pan.2017.07.007
Aronsson L, Andersson R, Ansari D. Intraductal papillary mucinous neoplasm of the pancreas - epidemiology, risk factors, diagnosis, and management. Scand J Gastroenterol. 2017;52(8):803-815. https://doi.org/10.1080/00365521.2017.1318948
Mino-Kenudson M, Fernández-del Castillo C, Baba Y, et al. Prognosis of invasive intraductal papillary mucinous neoplasm depends on histological and precursor epithelial subtypes. Gut. 2011;60(12):1712-1720. https://doi.org/10.1136/gut.2010.232272
Waters JA, Schnelldorfer T, Aguilar-Saavedra JR, et al. Survival after resection for invasive intraductal papillary mucinous neoplasm and for pancreatic adenocarcinoma: a multi-institutional comparison according to American Joint Committee on Cancer Stage. J Am Coll Surg. 2011;213(2):275-283. https://doi.org/10.1016/j.jamcollsurg.2011.04.003
Marchegiani G, Andrianello S, Dal Borgo C, et al. Adjuvant chemotherapy is associated with improved postoperative survival in specific subtypes of invasive intraductal papillary mucinous neoplasms (IPMN) of the pancreas: it is time for randomized controlled data. HPB (Oxford). 2019;21(5):596-603. https://doi.org/10.1016/j.hpb.2018.09.013
Caponi S, Vasile E, Funel N, et al. Adjuvant chemotherapy seems beneficial for invasive intraductal papillary mucinous neoplasms. Eur J Surg Oncol. 2013;39(4):396-403. https://doi.org/10.1016/j.ejso.2012.12.005
Turrini O, Waters JA, Schnelldorfer T, et al. Invasive intraductal papillary mucinous neoplasm: predictors of survival and role of adjuvant therapy. HPB (Oxford). 2010;12(7):447-455. https://doi.org/10.1111/j.1477-2574.2010.00196.x
McMillan MT, Lewis RS, Drebin JA, et al. The efficacy of adjuvant therapy for pancreatic invasive intraductal papillary mucinous neoplasm (IPMN). Cancer. 2016;122(4):521-533. https://doi.org/10.1002/cncr.29803
Mungo B, Croce C, Oba A, et al. Controversial Role of Adjuvant Therapy in Node-Negative Invasive Intraductal Papillary Mucinous Neoplasm [published online ahead of print, 2020 Aug 2]. Ann Surg Oncol. 2020; https://doi.org/10.1245/s10434-020-08916-6
Amini N, Habib JR, Blair A, et al. Invasive and Non-Invasive Progression after Resection of Non-Invasive Intraductal Papillary Mucinous Neoplasms [published online ahead of print, 2020 Nov 12]. Ann Surg. 2020; https://doi.org/10.1097/SLA.0000000000004488
Rodrigues C, Hank T, Qadan M, et al. Impact of adjuvant therapy in patients with invasive intraductal papillary mucinous neoplasms of the pancreas. Pancreatology. 2020;20(4):722-728. https://doi.org/10.1016/j.pan.2020.03.009
Psarelli E, Jackson R, et al. Patterns of Recurrence After Resection of Pancreatic Ductal Adenocarcinoma: A Secondary Analysis of the ESPAC-4 Randomized Adjuvant Chemotherapy Trial. JAMA Surg. 2019;154(11):1038–1048. https://doi.org/10.1001/jamasurg.2019.3337
Groot VP, Rezaee N, Wu W, et al. Patterns, Timing, and Predictors of Recurrence Following Pancreatectomy for Pancreatic Ductal Adenocarcinoma. Ann Surg. 2018;267(5):936-945. https://doi.org/10.1097/SLA.0000000000002234
Honselmann KC, Pergolini I, Castillo CF, et al. Timing But Not Patterns of Recurrence Is Different Between Node-negative and Node-positive Resected Pancreatic Cancer. Ann Surg. 2020 Aug;272(2):357-365. https://doi.org/10.1097/sla.0000000000003123.
Habib JR, Kinny-Köster B, Bou-Samra P, et al. Surgical decision making in pancreatic ductal adenocarcinoma: modeling prognosis following pancreatectomy in the era of induction and neoadjuvant chemotherapy. Ann Surg. (Accepted).
Amin MB, Greene FL, Edge SB, et al. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more "personalized" approach to cancer staging. CA Cancer J Clin. 2017;67(2):93–99.
Shimada K, Sakamoto Y, Sano T, Kosuge T, Hiraoka N. Invasive carcinoma originating in an intraductal papillary mucinous neoplasm of the pancreas: a clinicopathologic comparison with a common type of invasive ductal carcinoma. Pancreas. 2006;32(3):281-287. https://doi.org/10.1097/01.mpa.0000202955.33483.e2
Poultsides GA, Reddy S, Cameron JL, et al. Histopathologic basis for the favorable survival after resection of intraductal papillary mucinous neoplasm-associated invasive adenocarcinoma of the pancreas. Ann Surg. 2010;251(3):470-476. https://doi.org/10.1097/SLA.0b013e3181cf8a19
Winter JM, Jiang W, Basturk O, et al. Recurrence and Survival After Resection of Small Intraductal Papillary Mucinous Neoplasm-associated Carcinomas (≤20-mm Invasive Component): A Multi-institutional Analysis. Ann Surg. 2016;263(4):793-801. https://doi.org/10.1097/SLA.0000000000001319
Lewis R, Drebin JA, Callery MP, et al. A contemporary analysis of survival for resected pancreatic ductal adenocarcinoma. HPB (Oxford). 2013;15(1):49-60. https://doi.org/10.1111/j.1477-2574.2012.00571.x
Hayasaki A, Isaji S, Kishiwada M, et al. Survival Analysis in Patients with Pancreatic Ductal Adenocarcinoma Undergoing Chemoradiotherapy Followed by Surgery According to the International Consensus on the 2017 Definition of Borderline Resectable Cancer. Cancers (Basel). 2018;10(3):65. Published 2018 Mar 5. https://doi.org/10.3390/cancers10030065
Neoptolemos JP, Palmer DH, Ghaneh P, et al. Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): a multicentre, open-label, randomised, phase 3 trial. Lancet. 2017;389(10073):1011-1024. https://doi.org/10.1016/S0140-6736(16)32409-6
Marchegiani G, Mino-Kenudson M, Ferrone CR, et al. Patterns of Recurrence After Resection of IPMN: Who, When, and How?. Ann Surg. 2015;262(6):1108-1114. https://doi.org/10.1097/SLA.0000000000001008
Chari ST, Yadav D, Smyrk TC, et al. Study of recurrence after surgical resection of intraductal papillary mucinous neoplasm of the pancreas. Gastroenterology. 2002;123(5):1500-1507. https://doi.org/10.1053/gast.2002.36552
Hirono S, Shimizu Y, Ohtsuka T, et al. Recurrence patterns after surgical resection of intraductal papillary mucinous neoplasm (IPMN) of the pancreas; a multicenter, retrospective study of 1074 IPMN patients by the Japan Pancreas Society. J Gastroenterol. 2020;55(1):86-99. https://doi.org/10.1007/s00535-019-01617-2
Ciprani D, Morales-Oyarvide V, Qadan M, et al. An elevated CA 19-9 is associated with invasive cancer and worse survival in IPMN. Pancreatology. 2020;20(4):729-735. https://doi.org/10.1016/j.pan.2020.04.002
Wasif N, Bentrem DJ, Farrell JJ, et al. Invasive intraductal papillary mucinous neoplasm versus sporadic pancreatic adenocarcinoma: a stage-matched comparison of outcomes. Cancer. 2010;116(14):3369-3377. https://doi.org/10.1002/cncr.25070
Chari ST, Yadav D, Smyrk TC, et al. Study of recurrence after surgical resection of intraductal papillary mucinous neoplasm of the pancreas. Gastroenterology. 2002;123(5):1500-1507. https://doi.org/10.1053/gast.2002.36552
Maire F, Hammel P, Terris B, et al. Prognosis of malignant intraductal papillary mucinous tumours of the pancreas after surgical resection. Comparison with pancreatic ductal adenocarcinoma. Gut. 2002;51(5):717-722. https://doi.org/10.1136/gut.51.5.717
D'Angelica M, Brennan MF, Suriawinata AA, Klimstra D, Conlon KC. Intraductal papillary mucinous neoplasms of the pancreas: an analysis of clinicopathologic features and outcome. Ann Surg. 2004;239(3):400-408. https://doi.org/10.1097/01.sla.0000114132.47816.dd
Jang JY, Hwang DW, Kim MA, et al. Analysis of prognostic factors and a proposed new classification for invasive papillary mucinous neoplasms. Ann Surg Oncol. 2011;18(3):644-650. https://doi.org/10.1245/s10434-010-1331-6
Aronsson L, Marinko S, Ansari D, Andersson R. Adjuvant therapy in invasive intraductal papillary mucinous neoplasm (IPMN) of the pancreas: a systematic review. Ann Transl Med. 2019;7(22):689. https://doi.org/10.21037/atm.2019.10.37
Mungo B, Croce C, Oba A, et al. Controversial Role of Adjuvant Therapy in Node-Negative Invasive Intraductal Papillary Mucinous Neoplasm. Ann Surg Oncol. 2021;28(3):1533-1542. https://doi.org/10.1245/s10434-020-08916-6
Worni M, Akushevich I, Gloor B, et al. Adjuvant radiotherapy in the treatment of invasive intraductal papillary mucinous neoplasm of the pancreas: an analysis of the surveillance, epidemiology, and end results registry. Ann Surg Oncol. 2012;19(4):1316-1323. https://doi.org/10.1245/s10434-011-2088-2
Swartz MJ, Hsu CC, Pawlik TM, et al. Adjuvant chemoradiotherapy after pancreatic resection for invasive carcinoma associated with intraductal papillary mucinous neoplasm of the pancreas. Int J Radiat Oncol Biol Phys. 2010;76(3):839-844. https://doi.org/10.1016/j.ijrobp.2009.02.071
Funding
This work was supported by the Nikki Mitchell Foundation, the Ben and Rose Cole Charitable Pria Foundation, and the Faith Hope Love Gala. BK is supported by the German Research Foundation (KI 2437/2–1).
Author information
Authors and Affiliations
Contributions
Conception and study design: JRH, BK, NA, and CLW; acquisition of data: SS and AAJ; analysis and interpretation of data: JRH, JLC, EDT, EKF, RHH, JH, and CLW; review of imaging and pathologic slides: EKF, EDT, and RHH; manuscript drafting: JRH, BK, NA, SS, and AAJ; critically revised the manuscript: JLC, EDT, EKF, RHH, JH, and CLW; final approval of the manuscript version to be published: all authors.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests. The corresponding author had full access to all of the data and take full responsibility for the veracity of the data and statistical analysis.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Habib, J.R., Kinny-Köster, B., Amini, N. et al. Predictors, Patterns, and Timing of Recurrence Provide Insight into the Disease Biology of Invasive Carcinomas Arising in Association with Intraductal Papillary Mucinous Neoplasms. J Gastrointest Surg 26, 2311–2320 (2022). https://doi.org/10.1007/s11605-022-05428-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-022-05428-4