Abstract
Purpose
Relapse after complicated intra-abdominal infection (cIAI) remains common after treatment. The optimal antibiotic treatment duration for cIAIs is uncertain, especially in cases where source control is not achieved. We hypothesised that in patients with cIAIs, regardless of source control intervention, there would be a lower relapse rate with long-course antibiotics (28 days) compared with short course (≤ 10 days). We piloted a trial comparing ≤ 10-day with 28-day antibiotic treatment for cIAI.
Methods
A randomised controlled unblinded feasibility trial was conducted. Eligible participants were adult patients with a cIAI that were diagnosed ≤ 6 days prior to screening. Randomisation was to long-course (28 days) or short-course (≤10 days) antibiotic therapy. Choice of antibiotics was determined by the clinical team. Participants were followed up for 90 days. Primary outcomes were willingness of participants to be randomised and feasibility of trial procedures.
Results
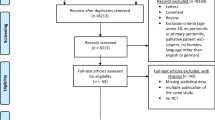
In total, 172 patients were screened, 84/172 (48.8%) were eligible, and 31/84 (36.9%) were randomised. Patients were assigned to either the short-course arm (18/31, 58.0%) or the long-course arm (13/31, 41.9%). One patient in the short-course arm withdrew after randomisation. In the short-course arm, 4/17 (23.5%) were treated for a cIAI relapse vs 0/13 (0.0%) relapses in the long-course arm. Protocol violations included deviations from protocol-assigned antibiotic duration and interruptions to antibiotic therapy.
Conclusions
This feasibility study identified opportunities to increase recruitment in a full trial. This study demonstrates completion of a randomised controlled trial to further evaluate if the optimum antibiotic duration for cIAIs is feasible.
Trial Registration
ClinicalTrials.gov Identifier: NCT03265834

Similar content being viewed by others
Data Availability
The datasets generated and analysed during the current study are available from the corresponding author on reasonable request.
References
Solomkin, J.S., et al., Diagnosis and management of complicated intra-abdominal infection in adults and children: guidelines by the Surgical Infection Society and the Infectious Diseases Society of America. Clin Infect Dis, 2010. 50(2): p. 133–64.
Brun-Buisson, C., et al., Incidence, risk factors, and outcome of severe sepsis and septic shock in adults. A multicenter prospective study in intensive care units. French ICU Group for Severe Sepsis. JAMA, 1995. 274(12): p. 968–74.
DeFrances, C.J., K.A. Cullen, and L.J. Kozak, National Hospital Discharge Survey: 2005 annual summary with detailed diagnosis and procedure data. Vital Health Stat 13, 2007(165): p. 1–209.
Sawyer, R.G., et al., Trial of short-course antimicrobial therapy for intraabdominal infection. N Engl J Med, 2015. 372(21): p. 1996–2005.
Montravers, P., et al., Short-course antibiotic therapy for critically ill patients treated for postoperative intra-abdominal infection: the DURAPOP randomised clinical trial. Intensive Care Med, 2018. 44(3): p. 300–310.
Wenzel, R.P. and M.B. Edmond, Antibiotics for abdominal sepsis. N Engl J Med, 2015. 372(21): p. 2062–3.
Ahmed S and the CABI Collaborative, Clinical Management of Complicated Intra-abdominal Infections in the United Kingdom, in 28th European Congress of Clinical Microbiology & Infectious Diseases. 2018: Madrid, Spain.
Billingham, S.A., A.L. Whitehead, and S.A. Julious, An audit of sample sizes for pilot and feasibility trials being undertaken in the United Kingdom registered in the United Kingdom Clinical Research Network database. BMC Med Res Methodol, 2013. 13: p. 104.
Nystrom, P.O., et al., Proposed definitions for diagnosis, severity scoring, stratification, and outcome for trials on intraabdominal infection. Joint Working Party of SIS North America and Europe. World J Surg, 1990. 14(2): p. 148–58.
Acknowledgements
Caroline Bedford, Lead Pharmacist for Clinical Trials, Leeds Teaching Hospitals Trust; Catherine Moriarty, Senior Research Sister, Leeds Teaching Hospitals NHS Trust; Sarah Brown, Medical Statistician, The University of Leeds; Shafaque Shaikh, University of Aberdeen at NHS Grampian.
Funding
Dr. Ahmed was awarded a 1-year clinical research fellowship by Leeds Cares, a charity based at Leeds Teaching Hospitals NHS Trust, UK.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception, design, material preparation, data collection and analysis. The first draft of the manuscript was written by Shadia Ahmed, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 37 kb)
Rights and permissions
About this article
Cite this article
Ahmed, S., Brown, R., Pettinger, R. et al. The CABI Trial: an Unblinded Parallel Group Randomised Controlled Feasibility Trial of Long-Course Antibiotic Therapy (28 Days) Compared with Short Course (≤ 10 Days) in the Prevention of Relapse in Adults Treated for Complicated Intra-Abdominal Infection. J Gastrointest Surg 25, 1045–1052 (2021). https://doi.org/10.1007/s11605-020-04545-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-020-04545-2