Abstract
Purpose
Shortening the duration of antibiotic therapy (ABT) is a key measure in antimicrobial stewardship. The optimal duration of ABT for treatment of postoperative intra-abdominal infections (PIAI) in critically ill patients is unknown.
Methods
A multicentre prospective randomised trial conducted in 21 French intensive care units (ICU) between May 2011 and February 2015 compared the efficacy and safety of 8-day versus 15-day antibiotic therapy in critically ill patients with PIAI. Among 410 eligible patients (adequate source control and ABT on day 0), 249 patients were randomly assigned on day 8 to either stop ABT immediately (n = 126) or to continue ABT until day 15 (n = 123). The primary endpoint was the number of antibiotic-free days between randomisation (day 8) and day 28. Secondary outcomes were death, ICU and hospital length of stay, emergence of multidrug-resistant (MDR) bacteria and reoperation rate, with 45-day follow-up.
Results
Patients treated for 8 days had a higher median number of antibiotic-free days than those treated for 15 days (15 [6–20] vs 12 [6–13] days, respectively; P < 0.0001) (Wilcoxon rank difference 4.99 days [95% CI 2.99–6.00; P < 0.0001). Equivalence was established in terms of 45-day mortality (rate difference 0.038, 95% CI − 0.013 to 0.061). Treatments did not differ in terms of ICU and hospital length of stay, emergence of MDR bacteria or reoperation rate, while subsequent drainages between day 8 and day 45 were observed following short-course ABT (P = 0.041).
Conclusion
Short-course antibiotic therapy in critically ill ICU patients with PIAI reduces antibiotic exposure. Continuation of treatment until day 15 is not associated with any clinical benefit.
Clinicaltrials.gov identifier
NCT01311765.
Similar content being viewed by others
This multicentre prospective randomised study evaluated the efficacy and safety of an 8-day course of antibiotic therapy in ICU patients treated for postoperative intra-abdominal infections. This regimen substantially reduced antibiotic exposure and no clinical benefit was observed when treatment was extended to 15 days. |
Introduction
Decreased duration of antibiotic therapy (ABT) is a fundamental antimicrobial stewardship measure [1]. It has recently been suggested that a short course of 4–5 days would be sufficient to cure mild-to-moderate healthcare-associated intra-abdominal infections [2]. However, the results to date are not sufficient to support a similar policy in critically ill patients such as those treated for severe postoperative intra-abdominal infection (PIAI), who require supportive care for organ dysfunctions, and those with high proportions of multidrug-resistant (MDR) bacteria [3,4,5,6,7,8].
In single-centre, retrospective, epidemiological studies analysing these ICU patients, the duration of ABT ranged between 7 and 15 days [9,10,11]. In addition, a significant increase in the duration of ABT was reported when MDR bacteria were cultured [10, 11]. The existing guidelines provide no relevant recommendations and suggest that the decision-making process should be based on clinician judgement and laboratory results [4, 7, 8].
We therefore conducted a randomised trial to compare 8-day and 15-day ABT regimens in a well-defined group of critically ill patients with PIAI, as confirmed by surgery and peritoneal specimen culture results.
Materials and methods
Study design and organisation
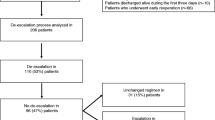
This multicentre, prospective, controlled, randomised, open-label trial was conducted in 21 French intensive care units (ICU) (Fig. 1 and electronic supplementary material [ESM] Figure-S1) between May 2011 and February 2015. The study was approved by the appropriate ethics committee (CPP Ile-de-France-I 2010-08-12392). Written informed consent was obtained from the patients or their legal representatives before study inclusion. In the event of impaired decision-making capacity without a legal representative available, the patient’s informed consent could be obtained after enrolment (emergency inclusion).
Study population
Patients were eligible for enrolment in the study if the following conditions were fulfilled: (1) admission to the ICU; (2) elapse of less than 24 h since surgery for PIAI (i.e. ≤ 60 days following an interventional procedure or any surgery performed in the peritoneal or retroperitoneal space showing gross pus or purulent effusion in the peritoneal cavity, or several collections); (3) adequate source control; (4) operative samples yielding positive microbiological cultures; (5) empirical ABT initiated within 24 h after completion of surgery; (6) written informed consent or emergency inclusion. The criteria for ineligibility are presented in the Methods-ESM.
Randomisation
Patients were included at day 0 (day of source control procedure). Randomisation was performed on day 8 to avoid any divergence between the arms before the two treatment strategies began to differ (Methods-ESM). Patients were randomly assigned on day 8, in a one/one ratio, to either cessation of ABT on day 8 (8-day arm) or continuation of antibiotics for another 7 days (15-day arm). The randomisation list was computer-generated, balanced by blocks of variables, of undisclosed size and stratified by centre. Allocation concealment was achieved using a centralised, secure, interactive web-response system accessible from each study centre (Cleanweb, Telemedicine Technologies S.A.S, Boulogne-Billancourt, France). Investigator blinding to arm assignment was not practicable, but all investigators were unaware of aggregate outcomes during the study.
Antibiotic treatments
Empirical ABT was recommended according to the French Guidelines for PIAI (Methods-ESM) [5]. However, ABT and management were left to the discretion of the attending physicians, including drug plasma monitoring and any adaptation considered necessary. Investigators were invited to convert the empirical regimen into narrow-spectrum treatment, based on culture results obtained within 48–72 h after surgery. It was planned to withdraw antibiotics at the end of allocated treatment. However, the physician in charge of the patient was the final arbiter of the decision when to stop ABT. If the physician did not follow the randomisation assignment, the reason for deviation was recorded. The use of procalcitonin to guide treatment decision making was left to the attending physician’s discretion.
Data collection
The following criteria were recorded from day 8 until day 45: death; time to discharge from ICU and hospital; need for reoperation or percutaneous drainage for any reason; need for another course of ABT for any reason; bacteraemia; microbiological recurrence in peritoneal samples, defined as culture of at least one of the initial causative micro-organisms from samples obtained from reoperation or percutaneous drainage (otherwise it was considered to be a superinfection). MDR bacteria were defined according to expert recommendations (Methods-ESM) [12]. Emergence of MDR bacteria in surveillance samples was assessed on a weekly basis from ICU admission to discharge. Organ failure (according to the SOFA score [13]) was evaluated on day 15 and day 28 in survivors not discharged from hospital.
Outcome measures
Recruitment difficulties led to a switch from two primary endpoints to one (Methods-ESM). The primary endpoint was the number of antibiotic-free days (i.e. number of days alive and antibiotic-free) from day 8 to day 28 (analysis of superiority); patients who died before day 28 were counted as no antibiotic-free days.
The key secondary endpoints were equivalence in terms of 45-day mortality and superiority analyses of death from any cause at day 28. Other secondary outcomes were: length of ICU and hospital stay; need for reoperation for any reason; need for additional drainage; superinfection or recurrent infection, assessed by microbiological sample; and another course of ABT for any reason.
Clinical failure was defined as the presence of any of these criteria between day 8 and day 45. Microbiological failure was defined as the presence of bacteria cultured from blood, pus or fluid collected from the abdominal cavity or abscesses collected from reoperation, or percutaneous drainage, infection recurrence or superinfection ≥ 48 h after stopping adequate ABT, or emergence of MDR micro-organisms in clinical isolates and surveillance samples assessed between day 1 and discharge from the ICU. The organ failure-free survival rate according to SOFA score was determined on day 15 and day 28.
Predefined subgroups of interest were: patients > 80 years; morbidly obese patients (BMI > 35 kg/m2); severe infection with SAPS II score > 40; [14] patients with bacteraemia during the first 3 days before and after the initial operation; patients with peritoneal samples yielding Pseudomonas aeruginosa or enterococci.
In post hoc analyses, evaluation of both strategies from the pragmatic point of view by incorporating the actual duration of antibiotic use was made using the concepts of desirability of outcome ranking (DOOR) and response adjusted for duration of antibiotic analyses (RADAR) [15]. Patients were divided into five mutually exclusive categories according to the postoperative course: (1) recovery without any complication; (2) recovery with additional ABT; (3) recovery with additional percutaneous drainage; (4) recovery with additional abdominal procedure; and (5) death. Probabilities of a better desirable outcome for a randomly selected patient, based on adjusted ranks (RADAR), were calculated for the two strategies.
Statistical analysis
The trial was designed to determine whether the 8-day antibiotic regimen was superior in terms of antibiotic use, assessed by the number of antibiotic-free days. Assuming 11.5 ± 5.5 (mean ± SD) antibiotic-free days for the 15-day arm (unpublished data from the Prorata trial, in the subgroup of patients with PIAI) [16], 122 patients/arm would provide 90% power at a two-sided α risk of 0.05 to detect a 20% (2.3 days) increase of the number of antibiotic-free days. We therefore planned to include and randomise 244 patients.
Categorical variables are reported as numbers (%). Continuous variables are expressed as mean ± SD and median [interquartile range (IQR)]. For all analyses (R software version 3.1), the significance alpha level was 0.05.
The primary endpoint was analysed according to the intention-to-treat (ITT) principle. The absolute difference between arms in terms of the numbers of antibiotic-free days was assessed using the two-sided Wilcoxon rank sum test. The center effect was evaluated in statistical analyses as a fixed effect for the principal and secondary outcomes. Multiple imputation methods (MICE) were used on missing values [17].
The equivalence in terms of 45-day mortality was analysed in the ITT population and repeated in the per-protocol population (defined as duration of treatment according to randomisation arm [± 1 day] and 48 h of washout before taking new antimicrobials). Equivalence was assessed by the 95% CI (± 10%) of the difference of the 45-day mortality rates. Overall survival was estimated using Kaplan–Meier methods and the log-rank test. All other secondary analyses were performed in the ITT population using Student’s t test and Fisher’s or Wilcoxon’s test as appropriate.
Linear and logistic regressions (exploratory analyses) were fitted in order to test the interaction of predefined subgroups of interest and the randomisation arm when explaining the following outcomes: antibiotic-free days, 45-day mortality and the emergence of MDR bacteria.
Results
Patient characteristics
Overall, 410 patients were included in the study and 249 patients were randomised on day 8 following surgery. After withdrawal of consent for 13 patients, data for 236 patients were analysed. The clinical and therapeutic characteristics at admission for PIAI are presented in Table 1 (ESM-Table-S1). Twenty-eight patients (14 patients in each arm) who underwent reoperation between day 0 and day 8 at a mean of 4 [2–6.5] days after inclusion were also included (Fig. 1). This group did not differ statistically significantly from the other randomised patients (ESM-Table-S2).
Fifty-six positive blood cultures were reported in 37 patients in the 3 days before and after inclusion. Overall, 591 micro-organisms were cultured from peritoneal samples (ESM-Table-S3). Empirical ABT was started on day 0 for PIAI in all patients, followed by a definitive antibiotic regimen based on microbiological results and susceptibility testing (ESM-Table-S4 and S5).
At day 8, 67 (26.9%) patients still had a SOFA score ≥ 3, and 72 (28.9%) patients had resumed oral feeding. The clinical characteristics at day 8 were similar in both arms except for a higher proportion of patients receiving mechanical ventilation in the 8-day arm (40/120 [35%] vs 24/116 [21%], p = 0.023) (Table 1 and ESM-Table-S6).
The allocated duration of ABT was followed in 92 (79%) patients in the 8-day arm and 95 (82%) patients in the 15-day arm (ESM-Results). Overall, 77 patients in the 8-day arm and 66 patients in the 15-day arm were included in the per-protocol analysis (ESM-Figure-S2).
Primary outcome
The number of antibiotic-free days between day 8 and day 28 was higher in the 8-day arm (median [IQR] 15 [6–20] days) than in the 15-day arm (12 [6–13] days) (difference in location estimates 4.99 days, 95% CI [2.99–6.00], p = 9.63 × 10−5) (Fig. 2 and ESM-Figure-S3).
Secondary outcomes
The 45-day mortality rates in the ITT population did not differ between the two arms (difference 3.8%, 95% CI [1.2–6.2]) (Table 2). The analysis was repeated in the per-protocol population: the 45-day mortality rates were 7/77 (9.1%) in the 8-day arm and 9/66 (13.6%) in the 15-day arm (difference 4.5%, 95% CI [1.13–7.96]).
Mortality rates from day 8 to day 28 in the ITT population were 9/120 (7.5%) in the 8-day arm and 13/116 (11.2%) in the 15-day arm (p = 0.37; OR 0.64 [in favour of the 8-day arm] 95% CI [0.23–1.7]). Similar results were observed in the PP population.
At day 28, the probability that the RADAR score would have been better with the 8-day regimen was 49.65% (95% CI [48.82–50.48]).
The log-rank test (p = 0.327) and Kaplan–Meier survival curves (Fig. 3) from randomisation until day 45 did not show any significant difference in terms of survival times between the two arms. Kaplan–Meier survival probability estimates at day 45 were about 0.89 for the 8-day arm and 0.85 for the 15-day arm.
Primary and secondary outcomes (two-sided analyses on ITT population). aDeceased patients have 0 days free of antibiotics; bdeceased patients leave the ICU on the day of death; cdeceased patients leave the hospital on the day of death; damong patients still hospitalised at day 15; eamong patients still hospitalised at day 28; famong those who underwent reoperation or additional drainage; gamong those who underwent surveillance samples or additional clinical isolates. Clinical and microbiological failures: see definitions in “Materials and methods”. IQR interquartile range, ICU intensive care unit, MDR bacteria multidrug-resistant bacteria
No significant difference between the arms was observed for emergence of MDR bacteria (Table 2).
Thirty-three patients in the 15-day arm and 40 patients in the 8-day arm were still hospitalised at day 45, and 16 patients (eight in each arm) were still in ICU. Other secondary outcomes are presented in Fig. 2.
Outcomes in predefined subgroups of interest
In order to evaluate differences of treatment effects in terms of antibiotic-free days, 45-day mortality and emergence of MDR bacteria in predefined specific subgroups of patients (ESM-Table 7), regressions were fitted and the interactions of each subgroup and the randomisation arm were tested (ESM-Tables 8a-8r).
Linear regression showed a significant association between antibiotic-free days and the interaction between randomisation arm and enterococcal infections (ESM-Table 8e) (estimate 4.26, 95% CI [1.07–7.46], p = 0.0092), showing a significantly higher mean ± SD number of antibiotic-free days in the group of patients with enterococcal infections (n = 106), (13.9 ± 5.5 vs 7.9 ± 6.3 days for the 8-day and 15-day arms, respectively).
Another association (but with a low level of evidence) was identified by logistic regression, namely that between the emergence of MDR bacteria and the interaction between randomisation arm and Pseudomonas infections (ESM-Table 8r: OR 0.20, 95% CI [0.039–0.99], p = 0.0491). In the group of patients with Pseudomonas infection, emergence of MDR bacteria was observed in 10/17 (59%) patients in the 15-day arm versus 4/19 (21%) in the 8-day arm.
Discussion
In this cohort of ICU patients with PIAI, in whom microbiological criteria were applied to diagnose intra-abdominal infection and who received appropriate initial empirical ABT, an 8-day antibiotic regimen reduced antibiotic exposure with equivalence in terms of 45-day mortality rates and no statistically significant difference in terms of 28-day mortality rate or clinical and bacteriological outcomes. The number of antibiotic-free days between day 8 (randomisation) and day 28 was higher in the 8-day arm, with an absolute difference of 3 days, corresponding to a 15% relative reduction in antibiotic exposure. No significant differences were observed for other outcomes, including mortality rate, infection recurrence, new ABT, and length of ICU and hospital stay.
The optimal duration of ABT for complicated intra-abdominal infections has been extensively debated, but data are lacking in ICU patients. Recent randomised trials evaluating new antibiotic agents for mild-to-moderate intra-abdominal infections have studied shortened durations of therapy [18,19,20,21], but limited numbers of cases of PIAI were included. Short-course treatments have been shown to be as effective as longer-course treatments for community-acquired peritonitis [2] and appendicitis [22], but not for PIAI in ICU patients. The 8-day course was chosen on the basis of the literature available at the time the protocol was designed. With growing confidence in less prolonged treatment, short-course therapy could be proposed in low-severity ICU cases. However, the need for additional drainage and bacteremia between day-8 and day-45 observed in the short-treatment arm could be considered to indicate a slightly higher failure rate of this regimen.
Our study population is representative of the ICU patients treated for PIAI [23,24,25]. The high proportion of exclusions before randomisation illustrates the uncertainties of management of these high-risk cases, including inadequacy of empirical ABT and premature death, with consequent recruitment difficulties. The proportion of inadequate empirical therapies in PIAI patients ranges between 10 and 90% [9, 11, 26], which justifies the recommendations for broad-spectrum ABT to target most of the potential micro-organisms [3,4,5,6,7,8]. Reoperation is common, performed in 15–51% of cases [9, 11, 26]. In addition, the early mortality rate can reach 70% in the first 10 days post surgery in patients with septic shock [27].
In our study, complication rates were similar in the two arms, with additional source control required in one-third of all patients. Surprisingly, there was a higher rate of percutaneous drainage in the 8-day arm. The attending physicians may have tended to screen for superinfection or recurrence more frequently in case of doubt when antibiotic administration was stopped early. Reoperation rates during the postoperative course of PIAI usually range between 5 and 51% of cases [9, 11, 26, 28]. The need for additional drainage is reported in 14–20% of patients [26].
Shortening the duration of ABT might help to contain the emergence of MDR bacteria in ICU patients [1, 29]. Surprisingly, we did not detect any between-group difference for the rates of emergence of MDR bacteria in the 15-day arm except for Pseudomonas aeruginosa, corresponding with previous reports from retrospective single-centre studies [24, 30]. However, the calculated sample size was not designed to assess this effect. Similarly, we did not observe any difference in the length of ICU stay between the two arms. However, length of stay depends on many factors not directly related to duration of ABT.
Several limitations need to be considered when interpreting our results. Firstly, the primary endpoint was not truly patient-centred in this high-risk population and was closely related to the intervention, almost directly determined by the study design. This criterion was previously used by Chastre et al. in their pivotal study assessing duration of ABT in ventilator-associated pneumonia [31]. In view of the extreme severity of illness in the study population, superiority in terms of the number of antibiotic-free days can be difficult to establish. Complications in this high-risk population could have increased the relapse rate or caused additional morbidity in the 8-day arm. Secondly, this study is underpowered to evaluate any clinically important effect on mortality. Recruitment difficulties due to strict randomisation criteria led to a switch from two primary endpoints to one, with a concern for interpreting the outcome results at 45-day. The unblinded design of our study could also be open to criticism. Allocation of the duration of ABT was delayed to day 8 to standardise patient follow-up, using rigorous criteria for outcome evaluation. Repeated monitoring of plasma antibiotic levels as well as various antibiotic administration regimens would have been difficult to perform under blinded conditions. Moreover, a double-blind design would have influenced the length of hospital stay for patients in the 15-day arm due to placebo infusion. A large subset of patients was excluded before randomisation, as indicated on the flow chart. Delayed randomisation at day 8, when the strategies in the two arms began to differ, allowed exclusion of cases with a high initial mortality rate, inadequacy of empirical ABT, and early reoperation. This important choice was made to avoid any focus on patients in whom no ABT strategy would have helped and the bias induced by their high mortality rate. This selection also explains the lower mortality rates observed in our study population. No centre effects were found in the statistical analysis. However, the strong differences in the number of recruited patients per centre might have induced bias results. Finally, no conclusions can be drawn concerning neutropenic or immunosuppressed patients, patients with recurrent intra-abdominal infection or patients with fungal infections.
In summary, an 8-day antibiotic regimen substantially reduced antibiotic exposure for ICU patients who develop microbiologically proven PIAI, while continuation of ABT from 8 to 15 days did not provide any clinical benefit. The issues of a higher number of cases of subsequent drainages and increased bacteremia following short-course ABT requires clarification. The lack of effect of the 8-day regimen on the emergence of MDR bacteria is another surprising finding that deserves attention. The clinical characteristics and causes of PIAI among patients in our cohort and the consistency of the results suggest that our conclusions may be applicable to many ICU patients with PIAI. This type of approach could help to reduce antibiotic use.
References
Barlam TF, Cosgrove SE, Abbo LM, MacDougall C, Schuetz AN, Septimus EJ, Srinivasan A, Dellit TH, Falck-Ytter YT, Fishman NO, Hamilton CW, Jenkins TC, Lipsett PA, Malani PN, May LS, Moran GJ, Neuhauser MM, Newland JG, Ohl CA, Samore MH, Seo SK, Trivedi KK (2016) Implementing an Antibiotic Stewardship Program: guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin Infect Dis 62(e51–77):27080992
Sawyer RG, Claridge JA, Nathens AB, Rotstein OD, Duane TM, Evans HL, Cook CH, O’Neill PJ, Mazuski JE, Askari R, Wilson MA, Napolitano LM, Namias N, Miller PR, Dellinger EP, Watson CM, Coimbra R, Dent DL, Lowry SF, Cocanour CS, West MA, Banton KL, Cheadle WG, Lipsett PA, Guidry CA, Popovsky K (2015) Trial of short-course antimicrobial therapy for intraabdominal infection. N Engl J Med 372(1996–2005):25992746
Chow AW, Evans GA, Nathens AB, Ball CG, Hansen G, Harding GK, Kirkpatrick AW, Weiss K, Zhanel GG (2010) Canadian practice guidelines for surgical intra-abdominal infections. Can J Infect Dis Med Microbiol 21(11–37):21358883
Solomkin JS, Mazuski JE, Bradley JS, Rodvold KA, Goldstein EJ, Baron EJ, O’Neill PJ, Chow AW, Dellinger EP, Eachempati SR, Gorbach S, Hilfiker M, May AK, Nathens AB, Sawyer RG, Bartlett JG (2010) Diagnosis and management of complicated intra-abdominal infection in adults and children: guidelines by the Surgical Infection Society and the Infectious Diseases Society of America. Clin Infect Dis 50(133–164):20034345
Montravers P, Dupont H, Leone M, Constantin JM, Mertes PM, Laterre PF, Misset B, Bru JP, Gauzit R, Sotto A, Brigand C, Hamy A, Tuech JJ (2015) Guidelines for management of intra-abdominal infections. Anaesth Crit Care Pain Med 34(117–130):25922057
Eckmann C (2016) Antimicrobial therapy of intra-abdominal infections in the era of multiresistance. Chirurg 87:26–33
Sartelli M, Catena F, Abu-Zidan FM, Ansaloni L, Biffl WL, Boermeester MA, Ceresoli M, Chiara O, Coccolini F, De Waele JJ, Di Saverio S, Eckmann C, Fraga GP, Giannella M, Girardis M, Griffiths EA, Kashuk J, Kirkpatrick AW, Khokha V, Kluger Y, Labricciosa FM, Leppaniemi A, Maier RV, May AK, Malangoni M, Martin-Loeches I, Mazuski J, Montravers P, Peitzman A, Pereira BM, Reis T, Sakakushev B, Sganga G, Soreide K, Sugrue M, Ulrych J, Vincent JL, Viale P, Moore EE (2017) Management of intra-abdominal infections: recommendations by the WSES 2016 consensus conference. World J Emerg Surg 12(22):28484510
Mazuski JE, Tessier JM, May AK, Sawyer RG, Nadler EP, Rosengart MR, Chang PK, O’Neill PJ, Mollen KP, Huston JM, Diaz JJ Jr, Prince JM (2017) The Surgical Infection Society Revised Guidelines on the Management of Intra-Abdominal Infection. Surg Infect (Larchmt) 18(1–76):28085573
Dupont H, Friggeri A, Touzeau J, Airapetian N, Tinturier F, Lobjoie E, Lorne E, Hijazi M, Regimbeau JM, Mahjoub Y (2011) Enterococci increase the morbidity and mortality associated with severe intra-abdominal infections in elderly patients hospitalized in the intensive care unit. J Antimicrob Chemother 66(2379–2385):21791444
Seguin P, Laviolle B, Chanavaz C, Donnio PY, Gautier-Lerestif AL, Campion JP, Malledant Y (2006) Factors associated with multidrug-resistant bacteria in secondary peritonitis: impact on antibiotic therapy. Clin Microbiol Infect 12(980–985):16961634
Montravers P, Augustin P, Grall N, Desmard M, Allou N, Marmuse JP, Guglielminotti J (2016) Characteristics and outcomes of anti-infective de-escalation during health care-associated intra-abdominal infections. Crit Care 20(83):27052675
Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, Harbarth S, Hindler JF, Kahlmeter G, Olsson-Liljequist B, Paterson DL, Rice LB, Stelling J, Struelens MJ, Vatopoulos A, Weber JT, Monnet DL (2012) Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect 18(268–281):21793988
Vincent JL, Moreno R, Takala J, Willatts S, De Mendonca A, Bruining H, Reinhart CK, Suter PM, Thijs LG (1996) The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med 22(707–710):8844239
Le Gall JR, Lemeshow S, Saulnier F (1993) A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. JAMA 270(2957–2963):8254858
Evans SR, Rubin D, Follmann D, Pennello G, Huskins WC, Powers JH, Schoenfeld D, Chuang-Stein C, Cosgrove SE, Fowler VG Jr, Lautenbach E, Chambers HF (2015) Desirability of Outcome Ranking (DOOR) and Response Adjusted for Duration of Antibiotic Risk (RADAR). Clin Infect Dis 61(800–806):26113652
Bouadma L, Luyt CE, Tubach F, Cracco C, Alvarez A, Schwebel C, Schortgen F, Lasocki S, Veber B, Dehoux M, Bernard M, Pasquet B, Regnier B, Brun-Buisson C, Chastre J, Wolff M (2010) Use of procalcitonin to reduce patients’ exposure to antibiotics in intensive care units (PRORATA trial): a multicentre randomised controlled trial. Lancet 375(463–474):20097417
van Buuren S, Groothuis-Oudshoorn K (2011) Mice: multivariate imputation by chained equations in R. J Stat Softw 45:1–67
De Waele JJ, Tellado JM, Weiss G, Alder J, Kruesmann F, Arvis P, Hussain T, Solomkin JS (2014) Efficacy and safety of moxifloxacin in hospitalized patients with secondary peritonitis: pooled analysis of four randomized phase III trials. Surg Infect (Larchmt) 15(567–575):24833256
Lucasti C, Popescu I, Ramesh MK, Lipka J, Sable C (2013) Comparative study of the efficacy and safety of ceftazidime/avibactam plus metronidazole versus meropenem in the treatment of complicated intra-abdominal infections in hospitalized adults: results of a randomized, double-blind. Phase II trial. J Antimicrob Chemother 68(1183–1192):23391714
Solomkin J, Hershberger E, Miller B, Popejoy M, Friedland I, Steenbergen J, Yoon M, Collins S, Yuan G, Barie PS, Eckmann C (2015) Ceftolozane/tazobactam plus metronidazole for complicated intra-abdominal infections in an era of multidrug resistance: results from a randomized, double-blind, phase 3 trial (ASPECT-cIAI). Clin Infect Dis 60(1462–1471):25670823
Solomkin JS, Ramesh MK, Cesnauskas G, Novikovs N, Stefanova P, Sutcliffe JA, Walpole SM, Horn PT (2014) Phase 2, randomized, double-blind study of the efficacy and safety of two dose regimens of eravacycline versus ertapenem for adult community-acquired complicated intra-abdominal infections. Antimicrob Agents Chemother 58(1847–1854):24342651
van Rossem CC, Schreinemacher MH, van Geloven AA, Bemelman WA (2016) Antibiotic duration after laparoscopic appendectomy for acute complicated appendicitis. JAMA Surg 151(323–329):26580850
De Waele J, Lipman J, Sakr Y, Marshall JC, Vanhems P, Barrera Groba C, Leone M, Vincent JL (2014) Abdominal infections in the intensive care unit: characteristics, treatment and determinants of outcome. BMC Infect Dis 14(420):25074742
Montravers P, Dufour G, Guglielminotti J, Desmard M, Muller C, Houissa H, Allou N, Marmuse JP, Augustin P (2015) Dynamic changes of microbial flora and therapeutic consequences in persistent peritonitis. Crit Care 19(70):25887649
Sartelli M, Catena F, Ansaloni L, Coccolini F, Corbella D, Moore EE, Malangoni M, Velmahos G, Coimbra R, Koike K, Leppaniemi A, Biffl W, Balogh Z, Bendinelli C, Gupta S, Kluger Y, Agresta F, Di Saverio S, Tugnoli G, Jovine E, Ordonez CA, Whelan JF, Fraga GP, Gomes CA, Pereira GA, Yuan KC, Bala M, Peev MP, Ben-Ishay O, Cui Y, Marwah S, Zachariah S, Wani I, Rangarajan M, Sakakushev B, Kong V, Ahmed A, Abbas A, Gonsaga RA, Guercioni G, Vettoretto N, Poiasina E, Diaz-Nieto R, Massalou D, Skrovina M, Gerych I, Augustin G, Kenig J, Khokha V, Trana C, Kok KY, Mefire AC, Lee JG, Hong SK, Lohse HA, Ghnnam W, Verni A, Lohsiriwat V, Siribumrungwong B, El Zalabany T, Tavares A, Baiocchi G, Das K, Jarry J, Zida M, Sato N, Murata K, Shoko T, Irahara T, Hamedelneel AO, Naidoo N, Adesunkanmi AR, Kobe Y, Ishii W, Oka K, Izawa Y, Hamid H, Khan I, Attri A, Sharma R, Sanjuan J, Badiel M, Barnabe R (2014) Complicated intra-abdominal infections worldwide: the definitive data of the CIAOW Study. World J Emerg Surg 9(37):24883079
Seguin P, Fedun Y, Laviolle B, Nesseler N, Donnio PY, Malledant Y (2010) Risk factors for multidrug-resistant bacteria in patients with post-operative peritonitis requiring intensive care. J Antimicrob Chemother 65(342–346):20008043
Riche FC, Dray X, Laisne MJ, Mateo J, Raskine L, Sanson-Le Pors MJ, Payen D, Valleur P, Cholley BP (2009) Factors associated with septic shock and mortality in generalized peritonitis: comparison between community-acquired and postoperative peritonitis. Crit Care 13(R99):19552799
Jung B, Molinari N, Nasri M, Hajjej Z, Chanques G, Jean-Pierre H, Panaro F, Jaber S (2013) Procalcitonin biomarker kinetics fails to predict treatment response in perioperative abdominal infection with septic shock. Crit Care 17(R255):24156734
Goldmann DA, Weinstein RA, Wenzel RP, Tablan OC, Duma RJ, Gaynes RP, Schlosser J, Martone WJ (1996) Strategies to prevent and control the emergence and spread of antimicrobial-resistant microorganisms in hospitals. A challenge to hospital leadership. JAMA 275(234–240):8604178
Augustin P, Tran-Dinh A, Valin N, Desmard M, Crevecoeur MA, Muller-Serieys C, Woerther PL, Marmuse JP, Bronchard R, Montravers P (2013) Pseudomonas aeruginosa post-operative peritonitis: clinical features, risk factors, and prognosis. Surg Infect (Larchmt) 14(297–303):23672242
Chastre J, Wolff M, Fagon JY, Chevret S, Thomas F, Wermert D, Clementi E, Gonzalez J, Jusserand D, Asfar P, Perrin D, Fieux F, Aubas S (2003) Comparison of 8 vs 15 days of antibiotic therapy for ventilator-associated pneumonia in adults: a randomized trial. JAMA 290(2588–2598):14625336
Acknowledgements
We would like to thank all physicians and nurses from all study sites for their help and support; and Isabelle Hoffmann, Ferial Toumi, Cyndie Nilusmas and Nadia Ettalhaoui (Clinical Research Unit, CHU Bichat-Claude Bernard) for their assistance in monitoring and study management.
Clinical investigators of the DURAPOP Trial Group (named with permission): CHU Bichat-Claude Bernard, Paris: Philippe Montravers MD, PhD; Regis Bronchard MD; Mathieu Desmard MD, PhD; CHU Amiens, Amiens: Herve Dupont MD, PhD; Melanie Levrard MD, Yazine Mahjoub MD, PhD; CHU Angers, Angers: Sigismond lasocki MD, PhD; Soizic Gergaud MD, Thomas Gaillard MD; CH Victor Dupouy, Argenteuil: Gaetan Plantefeve MD; Olivier Pajot MD; CHU Besançon, Besançon: Gilles Blasco MD; Emmanuel Samain MD, PhD; Guillaume Besch MD; Sebastien Pily-Floury MD, PhD; CHU Beaujon, Clichy: Catherine Paugam MD, PhD; Sebastien Pease MD, Paer Abback MD; CHU Dijon, Dijon: Claude Girard MD, PhD; CHU Grenoble, Grenoble: Jean-Francois Payen MD, PhD; Marie-Christine Herault MD; CHU Montpellier, Montpellier: Samir Jaber MD, PhD; Boris Jung MD, PhD; Jean-Marc Delay MD; CH Mulhouse, Mulhouse: Josette Gally MD; CHU Brabois, Nancy: Claude Meistelman MD, PhD; Jean-François Perrier MD; CHU Nantes, Nantes: Karim Asehnoune MD, PhD; Raphael Cinotti MD; CHU Cochin, Paris: Antoine Tesniere MD, PhD; Alexandre Mignon MD, PhD; CHU St Antoine, Paris: Thomas Lescot MD, PhD; Nouria Belhadj-Tahar MD; Marc Beaussier MD, PhD; CHU Lyon Sud, Pierre Bénite: Alain Lepape MD; Vincent Piriou MD, PhD; Florent Wallet MD; Candice Tassin MD; CHU Reims, Reims: Joel Cousson MD; Pascal Raclot MD; Thierry Floch MD; CHU Rennes, Rennes: Philippe Seguin MD, PhD; Yoann Launey MD, PhD; CHU Rouen, Rouen: Benoit Veber MD, PhD; Philippe Gouin MD; Thomas Clavier MD; CHU St-Etienne, St-Etienne: Christian Auboyer MD, PhD; CHRU Strasbourg, Strasbourg: Olivier Collanges MD, PhD; CH Intercommunal Villeneuve-St-George, Villeneuve-St-George: Jean-François Georger MD.
Funding
This work was supported by the Programme Hospitalier de Recherche Clinique (PHRC) National, 2009 (AOM 09024), funded by the French Ministry of Health. The sponsor was the Direction de la Recherche Clinique de l’Assistance Publique des Hôpitaux de Paris (France). Funding/Support and Role of Funder/Sponsor. The manuscript was prepared, reviewed and approved by the authors with no intervention from the sponsor, who also did not influence the decision to submit the manuscript for publication.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflicts of interest
Dr. Montravers reports personal fees and non-financial support from Astellas, AstraZeneca, Basilea, Bayer, Cubist, Menarini, MSD, Parexel, Pfizer, Tetraphase and The Medicines Company unrelated to the submitted work. Dr. Lescot reports grants, personal fees and non-financial support from Baxter, personal fees and non-financial support from Fresenius and personal fees from MSD unrelated to the submitted work. Dr. Paugam reports personal fees from MSD, Astellas and Baxter-Gambro unrelated to the submitted work. Dr. Lepape reports personal fees from Biomerieux unrelated to the submitted work. Dr. Blasco reports personal fees and non-financial support from Baxter, Astellas, Pfizer, MSD and Gilead unrelated to the submitted work. Dr. Asehnoune reports personal fees from LFB, Fresenius and Baxter unrelated to the submitted work. Dr. Jaber reports personal fees from Drager, Hamilton, Maquet and Fisher-Paykel unrelated to the submitted work. Dr. Lasocki reports research grants from Vifor Pharma and grants from LFB and Astellas unrelated to the submitted work. Dr. Dupont reports personal fees from Astellas, Pfizer, Gilead, Astrazeneca, Novartis, Merck, Cubist and and Paratek unrelated to the submitted work. No other disclosures were reported.
Additional information
DURAPOP Trial Group Investigators are listed in the Acknowledgements section.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Montravers, P., Tubach, F., Lescot, T. et al. Short-course antibiotic therapy for critically ill patients treated for postoperative intra-abdominal infection: the DURAPOP randomised clinical trial. Intensive Care Med 44, 300–310 (2018). https://doi.org/10.1007/s00134-018-5088-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-018-5088-x