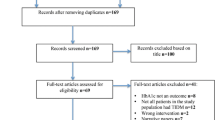
Abstract
Introduction
As obesity prevalence grows in the USA, metabolic syndrome is becoming increasingly more common. Current theories propose that insulin resistance is responsible for the hypertension, dyslipidemia, type II diabetes mellitus (T2DM), and low HDL that comprise metabolic syndrome. Bariatric surgery is one potential treatment, and its effects include permanently altering the patient’s physiology and glucose regulation. Consequently, patients with T2DM who undergo bariatric surgery often experience tighter glucose control or remission of their T2DM altogether. This meta-analysis aims to explore the physiologic mechanisms underlying T2DM remission following bariatric surgery, which demonstrates effects that could lead to expansion of the NIH criteria for bariatric surgery candidates.
Methods
A comprehensive search was conducted in PubMed and Scopus. Two independent reviewers conducted title, abstract, and full text review of papers that met inclusion criteria. Papers that measured hormone levels before and after bariatric surgery were included in the meta-analysis. Weighted means and standard deviations were calculated for preoperative and postoperative GLP-1, GIP, ghrelin, and glucagon.
Results
Total postprandial GLP-1 increased following bariatric surgery, which correlated with improvements in measures of glycemic control. Fasting GLP-1, fasting GIP, total postprandial GIP, total fasting ghrelin, and fasting glucagon all decreased, but all changes in hormones evaluated failed to reach statistical significance. Studies also demonstrated changes in hepatic and peripancreatic fat, inflammatory markers, miRNA, and gut microbiota following bariatric surgery.
Conclusion
While this meta-analysis sheds light on possible mechanisms, further studies are necessary to determine the dominant mechanism underlying remission of T2DM following bariatric surgery.


Similar content being viewed by others
References
Fryar CD, Carroll MD, Ogden CL. Prevalence of overweight, obesity, and extreme obesity among adults aged 20 and over: United States, 1960–1962 through 2011–2014. Natl Cent Heal Stat Data, Heal E-Stats 2016. https://www.cdc.gov/nchs/data/hestat/obesity_adult_13_14/obesity_adult_13_14.htm (accessed September 18, 2018).
Aguilar M, Bhuket T, Torres S, Liu B, Wong RJ. Prevalence of the Metabolic Syndrome in the United States, 2003-2012. JAMA 2015;313:1973. https://doi.org/10.1001/jama.2015.4260.
Müller-Wieland D, Kotzka J, Knebel B, Krone W. Metabolic syndrome and hypertension: pathophysiology and molecular basis of insulin resistance. Basic Res Cardiol 1998;93 Suppl 2:131–4.
Courcoulas A, Pories W. Potential Candidates for Bariatric Surgery | NIDDK. Natl Institutes Heal 2016. https://www.niddk.nih.gov/health-information/weight-management/bariatric-surgery/potential-candidates (accessed September 9, 2018).
Buchwald H, Avidor Y, Braunwald E, Jensen MD, Pories W, Fahrbach K, et al. Bariatric Surgery. JAMA 2004;292:1724. https://doi.org/10.1001/jama.292.14.1724.
Sjöström L, Lindroos A-K, Peltonen M, Torgerson J, Bouchard C, Carlsson B, et al. Lifestyle, Diabetes, and Cardiovascular Risk Factors 10 Years after Bariatric Surgery. N Engl J Med 2004;351:2683–93. https://doi.org/10.1056/NEJMoa035622.
Cummings DE, Overduin J, Foster-Schubert KE, Carlson MJ. Role of the bypassed proximal intestine in the anti-diabetic effects of bariatric surgery. Surg Obes Relat Dis 2007;3:109–15. https://doi.org/10.1016/j.soard.2007.02.003.
Cummings DE, Overduin J, Foster-Schubert KE. Gastric Bypass for Obesity: Mechanisms of Weight Loss and Diabetes Resolution. J Clin Endocrinol Metab 2004;89:2608–15. https://doi.org/10.1210/jc.2004-0433.
Colquitt JL, Pickett K, Loveman E, Frampton GK. Surgery for weight loss in adults. Cochrane Database Syst Rev 2014. https://doi.org/10.1002/14651858.CD003641.pub4.
Rubino F, Nathan DM, Eckel RH, Schauer PR, Alberti KGMM, Zimmet PZ, et al. Metabolic Surgery in the Treatment Algorithm for Type 2 Diabetes: A Joint Statement by International Diabetes Organizations. Surg Obes Relat Dis 2016;12:1144–62. https://doi.org/10.1016/j.soard.2016.05.018.
Gloy VL, Briel M, Bhatt DL, Kashyap SR, Schauer PR, Mingrone G, et al. Bariatric surgery versus non-surgical treatment for obesity: a systematic review and meta-analysis of randomised controlled trials. BMJ 2013;347:f5934. https://doi.org/10.1136/bmj.f5934.
Ford ES. Risks for all-cause mortality, cardiovascular disease, and diabetes associated with the metabolic syndrome: a summary of the evidence. Diabetes Care 2005;28:1769–78. https://doi.org/10.2337/DIACARE.28.7.1769.
Galassi A, Reynolds K, He J. Metabolic Syndrome and Risk of Cardiovascular Disease: A Meta-Analysis. Am J Med 2006;119:812–9. https://doi.org/10.1016/J.AMJMED.2006.02.031.
Gami AS, Witt BJ, Howard DE, Erwin PJ, Gami LA, Somers VK, et al. Metabolic Syndrome and Risk of Incident Cardiovascular Events and Death: A Systematic Review and Meta-Analysis of Longitudinal Studies. J Am Coll Cardiol 2007;49:403–14. https://doi.org/10.1016/J.JACC.2006.09.032.
Hanley AJG, Williams K, Festa A, Wagenknecht LE, D’Agostino RB, Haffner SM. Liver Markers and Development of the Metabolic Syndrome. Diabetes 2005;54:3140–7. https://doi.org/10.2337/DIABETES.54.11.3140.
Hamaguchi M, Kojima T, Takeda N, Nakagawa T, Taniguchi H, Fujii K, et al. The Metabolic Syndrome as a Predictor of Nonalcoholic Fatty Liver Disease. Ann Intern Med 2005;143:722. https://doi.org/10.7326/0003-4819-143-10-200511150-00009.
Marceau P, Biron S, Hould F-S, Marceau S, Simard S, Thung SN, et al. Liver Pathology and the Metabolic Syndrome X in Severe Obesity. J Clin Endocrinol Metab 1999;84:1513–7. https://doi.org/10.1210/jcem.84.5.5661.
Chen J, Muntner P, Hamm LL, Jones DW, Batuman V, Fonseca V, et al. The Metabolic Syndrome and Chronic Kidney Disease in U.S. Adults. Ann Intern Med 2004;140:167. https://doi.org/10.7326/0003-4819-140-3-200402030-00007.
Pasquali R, Gambineri A, Anconetani B, Vicennati V, Colitta D, Caramelli E, et al. The natural history of the metabolic syndrome in young women with the polycystic ovary syndrome and the effect of long-term oestrogen-progestagen treatment. Clin Endocrinol (Oxf) 1999;50:517–27. https://doi.org/10.1046/j.1365-2265.1999.00701.x.
Vgontzas AN, Papanicolaou DA, Bixler EO, Hopper K, Lotsikas A, Lin H-M, et al. Sleep Apnea and Daytime Sleepiness and Fatigue: Relation to Visceral Obesity, Insulin Resistance, and Hypercytokinemia. J Clin Endocrinol Metab 2000;85:1151–8. https://doi.org/10.1210/jcem.85.3.6484.
Choi HK, Ford ES. Prevalence of the Metabolic Syndrome in Individuals with Hyperuricemia. Am J Med 2007;120:442–7. https://doi.org/10.1016/J.AMJMED.2006.06.040.
Mechanick J, Youdim A, Jones D, Timothy Garvey W, Hurley D, Molly McMahon M, et al. Clinical Practice Guidelines for the Perioperative Nutritional, Metabolic, and Nonsurgical Support of the Bariatric Surgery Patient—2013 Update: Cosponsored by American Association of Clinical Endocrinologists, The Obesity Society, and American Society for Metabolic & Bariatric Surgery. SOARD 2013;9:159–91. https://doi.org/10.1016/j.soard.2012.12.010.
Nosso G, Griffo E, Cotugno M, Saldalamacchia G, Lupoli R, Pacini G, et al. Comparative Effects of Roux-en-Y Gastric Bypass and Sleeve Gastrectomy on Glucose Homeostasis and Incretin Hormones in Obese Type 2 Diabetic Patients: A One-Year Prospective Study. Horm Metab Res 2016;48:312–7. https://doi.org/10.1055/s-0041-111505.
Umeda LM, Silva EA, Carneiro G, Arasaki CH, Geloneze B, Zanella MT. Early improvement in glycemic control after bariatric surgery and its relationships with insulin, GLP-1, and glucagon secretion in type 2 diabetic patients. Obes Surg 2011;21:896–901. https://doi.org/10.1007/s11695-011-0412-3.
Vetter M, Wadden T, Teff K, Khan Z, Carvajal R, Ritter S, et al. GLP-1 Plays a Limited Role in Improved Glycemia Shortly After Roux-en-Y Gastric Bypass: A Comparison With Intensive Lifestyle Modification. Diabetes 2015;64:434–46. https://doi.org/10.2337/db14-055.
De Jonge C, Rensen SS, Verdam FJ, Vincent RP, Bloom SR, Buurman WA, et al. Endoscopic Duodenal–Jejunal Bypass Liner Rapidly Improves Type 2 Diabetes. Obes Surg 2013;23:1354–60. https://doi.org/10.1007/s11695-013-0921-3.
Martinussen C, Bojsen-Møller KN, Dirksen C, Jacobsen SH, Jørgensen NB, Kristiansen VB, et al. Immediate enhancement of first-phase insulin secretion and unchanged glucose effectiveness in patients with type 2 diabetes after Roux-en-Y gastric bypass. Am J Physiol - Endocrinol Metab 2015;308.
Baggio LL, Drucker DJ. Biology of Incretins: GLP-1 and GIP. Gastroenterology 2007;132:2131–57. https://doi.org/10.1053/j.gastro.2007.03.054.
Holst JJ. The Physiology of Glucagon-like Peptide 1. Physiol Rev 2007;87:1409–39. https://doi.org/10.1152/physrev.00034.2006.
Fellici AC, Lambert G, Lima MMO, Pareja JC, Rodovalho S, Chaim EA, et al. Surgical Treatment of Type 2 Diabetes in Subjects with Mild Obesity: Mechanisms Underlying Metabolic Improvements. Obes Surg 2015;25:36–44. https://doi.org/10.1007/s11695-014-1377-9.
Kashyap SR, Daud S, Kelly KR, Gastaldelli A, Win H, Brethauer S, et al. Acute effects of gastric bypass versus gastric restrictive surgery on beta-cell function and insulinotropic hormones in severely obese patients with type 2 diabetes. Int J Obes (Lond) 2010;34:462–71. https://doi.org/10.1038/ijo.2009.254.
Khoo CM, Muehlbauer MJ, Stevens RD, Pamuklar Z, Chen J, Newgard CB, et al. Postprandial metabolite profiles reveal differential nutrient handling after bariatric surgery compared with matched caloric restriction. Ann Surg 2014;259:687–93. https://doi.org/10.1097/SLA.0b013e318296633f.
Lee W-J, Chen C-Y, Chong K, Lee Y-C, Chen S-C, Lee S-D. Changes in postprandial gut hormones after metabolic surgery: a comparison of gastric bypass and sleeve gastrectomy. Surg Obes Relat Dis 2011;7:683–90. https://doi.org/10.1016/j.soard.2011.07.009.
Malin SK, Samat A, Wolski K, Abood B, Pothier CE, Bhatt DL, et al. Improved acylated ghrelin suppression at 2 years in obese patients with type 2 diabetes: effects of bariatric surgery vs standard medical therapy. Int J Obes 2014;38:364–70. https://doi.org/10.1038/ijo.2013.196.
Pournaras DJ, Osborne A, Hawkins SC, Vincent RP, Mahon D, Ewings P, et al. Remission of type 2 diabetes after gastric bypass and banding: mechanisms and 2 year outcomes. Ann Surg 2010;252:966–71. https://doi.org/10.1097/SLA.0b013e3181efc49a.
Salinari S, Bertuzzi A, Asnaghi S, Guidone C, Manco M, Mingrone G. First-Phase Insulin Secretion Restoration and Differential Response to Glucose Load Depending on the Route of Administration in Type 2 Diabetic Subjects After Bariatric Surgery. Diabetes Care 2009;32:375–80. https://doi.org/10.2337/dc08.
Rodrigues MR da S, Santo MA, Favero GM, Vieira EC, Artoni RF, Nogaroto V, et al. Metabolic surgery and intestinal gene expression: digestive tract and diabetes evolution considerations. World J Gastroenterol 2015;21:6990–8.
Bradnova O, Kyrou I, Hainer V, Vcelak J, Halkova T, Sramkova P, et al. Laparoscopic Greater Curvature Plication in Morbidly Obese Women with Type 2 Diabetes: Effects on Glucose Homeostasis, Postprandial Triglyceridemia and Selected Gut Hormones. Obes Surg 2014;24:718–26. https://doi.org/10.1007/s11695-013-1143-4.
Jimenez A, Casamitjana R, Flores L, Viaplana J, Corcelles R, Lacy A, et al. Long-Term Effects of Sleeve Gastrectomy and Roux-en-Y Gastric Bypass Surgery on Type 2 Diabetes Mellitus in Morbidly Obese Subjects. AnnSurg 2012;256:1023–9. https://doi.org/10.1097/SLA.0b013e318262ee6b
Jimenez A, Casamitjana R, Flores L, MD P, Delgado S, Lacy A, et al. GLP-1 and the Long-Term Outcome of Type 2 Diabetes Mellitus After Roux-en-Y Gastric Bypass Surgery in Morbidly Obese Subjects. Ann Surg 2013;257:894–9. https://doi.org/10.1097/SLA.0b013e31826b8603.
Thorens B. Glucagon-like peptide-1 and control of insulin secretion. Diabete Metab 1995;21:311–8.
Seino Y, Fukushima M, Yabe D. GIP and GLP-1, the two incretin hormones: Similarities and differences. J Diabetes Investig 2010;1:8–23. https://doi.org/10.1111/j.2040-1124.2010.00022.x.
Skrha J, Hilgertová J, Jarolímková M, Kunešová M, Hill M. Meal test for glucose-dependent insulinotropic peptide (GIP) in obese and type 2 diabetic patients. Physiol Res 2010;59:749–55.
Schwartz MW, Woods SC, Porte D, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature 2000;404:661–71. https://doi.org/10.1038/35007534.
Tschöp M, Smiley DL, Heiman ML. Ghrelin induces adiposity in rodents. Nature 2000;407:908–13. https://doi.org/10.1038/35038090.
Jerlhag E, Egecioglu E, Dickson SL, Douhan A, Svensson L, Engel JA. Ghrelin administration into tegmental areas stimulates locomotor activity and increases extracellular concentration of dopamine in the nucleus accumbens. Addict Biol 2007;12:6–16. https://doi.org/10.1111/j.1369-1600.2006.00041.x.
Jerlhag E, Egecioglu E, Dickson SL, Andersson M, Svensson L, Engel JA. Ghrelin stimulates locomotor activity and accumbal dopamine-overflow via central cholinergic systems in mice: implications for its involvement in brain reward. Addict Biol 2006;11:45–54. https://doi.org/10.1111/j.1369-1600.2006.00002.x.
Liljenquist JE, Bomboy JD, Lewis SB, Sinclair-Smith BC, Felts PW, Lacy WW, et al. Effects of Glucagon on Lipolysis and Ketogenesis in Normal and Diabetic Men. J Clin Invest 1974;53:190–7. https://doi.org/10.1172/JCI107537.
Fasanmade OA, Odeniyi IA, Ogbera AO. Diabetic ketoacidosis: diagnosis and management. Afr J Med Med Sci 2008;37:99–105.
Immonen H, Hannukainen JC, Iozzo P, Soinio M, Salminen P, Saunavaara V, et al. Effect of bariatric surgery on liver glucose metabolism in morbidly obese diabetic and non-diabetic patients. J Hepatol 2014;60:377–83. https://doi.org/10.1016/j.jhep.2013.09.012.
Honka H, Koffert J, Hannukainen JC, Tuulari JJ, Karlsson HK, Immonen H, et al. The effects of bariatric surgery on pancreatic lipid metabolism and blood flow. J Clin Endocrinol Metab 2015;100:2015–23. https://doi.org/10.1210/jc.2014-4236.
Shih K-C, Janckila AJ, Lee W-J, Chou Y-C, Huang C-J, Kwok C-F, et al. Effects of bariatric weight loss surgery on glucose metabolism, inflammatory cytokines, and serum tartrate-resistant acid phosphatase 5a in obese Chinese adults. Clin Chim Acta 2016;453:197–202. https://doi.org/10.1016/j.cca.2015.11.004.
Lirun K, Sewe M, Yong W. A Pilot Study: The Effect of Roux-en-Y Gastric Bypass on the Serum MicroRNAs of the Type 2 Diabetes Patient. Obes Surg 2015;25:2386–92. https://doi.org/10.1007/s11695-015-1711-x.
Clemente-Postigo M, Roca-Rodriguez MDM, Camargo A, Ocaña-Wilhelmi L, Cardona F, Tinahones FJ. Lipopolysaccharide and lipopolysaccharide-binding protein levels and their relationship to early metabolic improvement after bariatric surgery. Surg Obes Relat Dis 2015;11:933–9. https://doi.org/10.1016/j.soard.2014.11.030.
Author information
Authors and Affiliations
Contributions
Sarah M. Russel, B.S.: methodology, formal analysis, investigation, writing–original draft, and writing–review and editing
Valentina Valle, M.D.: methodology, investigation, and writing–review and editing
Giuditta Spagni, M.D.: methodology, investigation, and writing–review and editing
Sarah Hamilton: investigation and writing–review and editing
Takshaka Patel, B.S., B.A.: investigation, writing–original draft, and writing–review and editing
Nurlan Abdukadyrov, B.S.: formal analysis and writing–review and editing
Yushen Dong, B.S.: formal analysis and writing–review and editing
Antonio Gangemi, M.D., F.A.C.S, F.A.S.M.B.S.: conceptualization, supervision, funding acquisition, methodology, and writing–review and editing
Ethics declarations
Conflict of Interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Russel, S.M., Valle, V., Spagni, G. et al. Physiologic Mechanisms of Type II Diabetes Mellitus Remission Following Bariatric Surgery: a Meta-analysis and Clinical Implications. J Gastrointest Surg 24, 728–741 (2020). https://doi.org/10.1007/s11605-019-04508-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-019-04508-2