Abstract
Background
Postoperative chemotherapy (CMT) or chemoradiotherapy (CRT) is commonly recommended for gastric cancer (GC) patients in order to improve survival. However, some factors that prevent patients from return to intended oncologic treatment (RIOT) may increase the risk of recurrence and decrease the survival benefits achieved with curative resection. The aim of this study was to determine the frequency and factors associated with inability to RIOT and their impact on survival.
Methods
This retrospective study included stage II/III GC patients treated with potentially curative gastrectomy. Patients who could return to intended oncologic treatment (RIOT group) and those who could not (inability to RIOT group) were analyzed.
Results
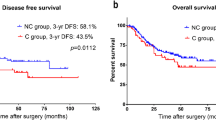
Of the 313 eligible GC patients, 89 (28.4%) and 85 (27.2%) patients receive CRT and CMT, respectively, representing a RIOT rate of 55.6%. The main reason was attributed to general poor performance status (30.2%), followed by surgical postoperative complications (POC) (20.1%). Older age, higher ASA, D1 lymphadenectomy, and major POC were related to inability to RIOT. Older age, neutrophil-lymphocyte ratio (NLR), and major POC were independent risk factors for inability to RIOT. Five-year DFS and OS were worse for the inability to RIOT group than for the RIOT group (p = 0.008 and p = 0.004, respectively). In multivariate analyses, absence of neoadjuvant therapy, total gastrectomy, pT3/T4, pN+, and inability to RIOT were associated with worse DFS. Type of gastrectomy, lymphadenectomy, pN status, Rx resection, and RIOT group were associated with OS.
Conclusion
Older age, high NLR, and major POC were risk factors for inability to RIOT. RIOT was an independent predictor of survival.


Similar content being viewed by others
References
Japanese Gastric Cancer A. Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric Cancer 2017;20:1-19.
Sakuramoto S, Sasako M, Yamaguchi T, et al. Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med 2007;357:1810-20.
Macdonald JS, Smalley SR, Benedetti J, et al. Chemoradiotherapy after Surgery Compared with Surgery Alone for Adenocarcinoma of the Stomach or Gastroesophageal Junction. New England Journal of Medicine 2001;345:725-730.
Bang YJ, Kim YW, Yang HK, et al. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): a phase 3 open-label, randomised controlled trial. Lancet 2012;379:315-21.
Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med 2006;355:11-20.
Aloia TA, Zimmitti G, Conrad C, et al. Return to intended oncologic treatment (RIOT): a novel metric for evaluating the quality of oncosurgical therapy for malignancy. J Surg Oncol 2014;110:107-14.
Zilberstein B, Malheiros C, Lourenco LG, et al. Brazilian consensus in gastric cancer: guidelines for gastric cancer in Brazil. Arq Bras Cir Dig 2013;26:2-6.
Tang L, Berlin J, Burgart L, et al. Protocol for the Examination of Specimens From Patients With Carcinoma of the Stomach. College of American Pathologists (CAP) 2014;3.3.0.0.
Ajani JA, In H, Sano T, et al. American Joint Committee on Cancer (AJCC). Cancer Staging Manual. 8th edition. Stomach. Springer 2017;17:203 - 220.
Doyle DJ, Garmon EH. American Society of Anesthesiologists Classification (ASA Class). StatPearls. Treasure Island FL: StatPearls Publishing LLC., 2017.
Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373-83.
Szor DJ, Roncon Dias A, Pereira MA, et al. Neutrophil-lymphocyte ratio is associated with prognosis in patients who underwent potentially curative resection for gastric cancer. Journal of Surgical Oncology 2018.
Dindo D, Demartines N, Clavien P-A. Classification of Surgical Complications: A New Proposal With Evaluation in a Cohort of 6336 Patients and Results of a Survey. Annals of Surgery 2004;240:205-213.
Macdonald JS, Fleming TR, Peterson RF, et al. Adjuvant chemotherapy with 5-FU, adriamycin, and mitomycin-C (FAM) versus surgery alone for patients with locally advanced gastric adenocarcinoma: A Southwest Oncology Group study. Ann Surg Oncol 1995;2:488-94.
Boku N. Chemotherapy for metastatic disease: review from JCOG trials. International Journal of Clinical Oncology 2008;13:196-200.
Al-Batran S-E, Homann N, Schmalenberg H, et al. Perioperative chemotherapy with docetaxel, oxaliplatin, and fluorouracil/leucovorin (FLOT) versus epirubicin, cisplatin, and fluorouracil or capecitabine (ECF/ECX) for resectable gastric or gastroesophageal junction (GEJ) adenocarcinoma (FLOT4-AIO): A multicenter, randomized phase 3 trial. Journal of Clinical Oncology 2017;35:4004-4004.
Lee J, Lim DH, Kim S, et al. Phase III trial comparing capecitabine plus cisplatin versus capecitabine plus cisplatin with concurrent capecitabine radiotherapy in completely resected gastric cancer with D2 lymph node dissection: the ARTIST trial. J Clin Oncol 2012;30:268-73.
Dikken JL, van Sandick JW, Maurits Swellengrebel HA, et al. Neo-adjuvant chemotherapy followed by surgery and chemotherapy or by surgery and chemoradiotherapy for patients with resectable gastric cancer (CRITICS). BMC Cancer 2011;11:329.
Cats A, Jansen EPM, van Grieken NCT, et al. Chemotherapy versus chemoradiotherapy after surgery and preoperative chemotherapy for resectable gastric cancer (CRITICS): an international, open-label, randomised phase 3 trial. Lancet Oncol 2018;19:616-628.
Karagkounis G, Squires MH, 3rd, Melis M, et al. Predictors and Prognostic Implications of Perioperative Chemotherapy Completion in Gastric Cancer. J Gastrointest Surg 2017;21:1984-1992.
Ramos M, Pereira MA, Dias AR, et al. Surgical outcomes of gastrectomy with D1 lymph node dissection performed for patients with unfavorable clinical conditions. Eur J Surg Oncol 2019;45:460-465.
Szor DJ, Dias AR, Pereira MA, et al. Prognostic Role of Neutrophil/Lymphocyte Ratio in Resected Gastric Cancer: A Systematic Review and Meta-analysis. Clinics 2018;73:e360.
Jin LX, Sanford DE, Squires MH, 3rd, et al. Interaction of Postoperative Morbidity and Receipt of Adjuvant Therapy on Long-Term Survival After Resection for Gastric Adenocarcinoma: Results From the U.S. Gastric Cancer Collaborative. Ann Surg Oncol 2016;23:2398-408.
Kubota T, Hiki N, Sano T, et al. Prognostic significance of complications after curative surgery for gastric cancer. Ann Surg Oncol 2014;21:891-8.
Aahlin EK, Olsen F, Uleberg B, et al. Major postoperative complications are associated with impaired long-term survival after gastro-esophageal and pancreatic cancer surgery: a complete national cohort study. BMC Surg 2016;16:32.
Goense L, Meziani J, Ruurda JP, et al. Impact of postoperative complications on outcomes after oesophagectomy for cancer. Br J Surg 2019;106:111-119.
Pucher PH, Aggarwal R, Qurashi M, et al. Meta-analysis of the effect of postoperative in-hospital morbidity on long-term patient survival. Br J Surg 2014;101:1499-508.
Ramos MFKP, Pereira MA, Barchi LC, et al. Duodenal fistula: The most lethal surgical complication in a case series of radical gastrectomy. Int J Surg 2018;53:366-370.
Ruspi L, Galli F, Pappalardo V, et al. Lymphadenectomy in elderly/high risk patients: should it be different? Transl Gastroenterol Hepatol 2017;2.
Wang S, Xu L, Wang Q, et al. Postoperative complications and prognosis after radical gastrectomy for gastric cancer: a systematic review and meta-analysis of observational studies. World J Surg Oncol 2019;17:52.
Vicente D, Ikoma N, Chiang YJ, et al. Preoperative Therapy for Gastric Adenocarcinoma is Protective for Poor Oncologic Outcomes in Patients with Complications After Gastrectomy. Ann Surg Oncol 2018;25:2720-2730.
Author information
Authors and Affiliations
Contributions
Marcus F.K.P. Ramos, Tiago B. de Castria, Marina A. Pereira: study design, data retrieval, statistical analysis, draft of the manuscript.
Andre R. Dias, Fernanda F Antonacio: data retrieval, review of the manuscript.
Bruno Zilberstein, Paulo M.G. Hoff, Ulysses Ribeiro Jr, Ivan Cecconello: critical analysis, review of the manuscript.
Corresponding author
Ethics declarations
The study was approved by the hospital ethics committee (NP993/16) and registered online (www.plataformabrasil.org.br). Informed consent of patients was waived because of the retrospective nature of the study.
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Oral presentation at Digestive Disease Week - Plenary Session, 2019, San Diego, USA
Electronic Supplementary Material
Supplementary Table 1
Postoperative complications for RIOT and inability to RIOT groups according to Clavien-Dindo classification. (DOCX 16 kb)
Supplementary Table 2
Adjuvant treatment trials and RIOT. (DOCX 13 kb)
Supplementary Table 3
Perioperative treatment trials and RIOT. (DOCX 14 kb)
Rights and permissions
About this article
Cite this article
Ramos, M.F.K.P., de Castria, T.B., Pereira, M.A. et al. Return to Intended Oncologic Treatment (RIOT) in Resected Gastric Cancer Patients. J Gastrointest Surg 24, 19–27 (2020). https://doi.org/10.1007/s11605-019-04462-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-019-04462-z