Abstract
Purpose
Three-dimensional (3D) visualization technology has been increasingly applied in patient-specific surgeries, but its value in residency training has not been determined. This study aimed to explore the value of 3D visualized pancreatic model in tumor evaluation and surgery planning for surgical trainees.
Methods
Eighty-eight surgical residents were randomized into two groups (computed tomography (CT) group and 3D group). Both groups began with a training on evaluating the resectability of pancreatic tumor, which was based on the NCCN clinical practice guidelines and practiced on a sample case. Then, they respectively learned the sample case either on 3D reconstruction visualization tables or CT images. Finally, both groups completed a same test consisting of two pancreatic cases with CT images as well as questionnaires.
Results
No differences were found in scores of the anatomy and diagnosis part, while mean scores for questions, associated with tumor staging and surgery planning, were consistently and significantly higher in the 3D group. In addition, participants in 3D group agreed that 3D technology was more beneficial in understanding and making pancreatic surgery planning.
Conclusion
The 3D visualization table may be a potential supplemental learning tool in building anatomy-image-surgery knowledge system and thus making surgery planning for surgical trainees.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
Introduction
Pancreatic cancer, one of the most lethal cancers, is characterized by aggressive invasion and early metastasis.1 Complete resection offers the only chance of relatively long-term survival. Success of pancreatectomy depends on detailed knowledge of tumor anatomy as well as its relationship with the adjacent tissues. Errors in surgery planning may raise risk of surgical failure and recurrence. Thus, precise evaluation of tumor resectability is crucial for pancreatic surgery planning.
With the rapid development of digital technologies, 3D visualization systems have shown increasing potential value in surgery and medical education. A series of studies indicated that 3D anatomy models have been widely used in surgical teaching, patient education, and preoperative planning.2,3,4,5,6 However, a 3D-based standardized surgical training for residents is relatively lacking. Depending on this, in this study, we developed a 3D training of pancreatic cancer via 3D real-time reconstruction multi-touch visualization table to evaluate its value among surgical trainees in the anatomy-image-surgery knowledge system building and surgery planning.
Materials and Methods
Generating 3D Simulation Models with 3D Technology
In this study, 3D reconstructions were created by a 3D multi-touch visualization table (MVT), which was introduced by Sectra in 2010 at the Radiological Society of North America. CT data of three pancreatic cancer cases were imported into the MVT first, and real-time 3D simulation models were consequently reconstructed. All the reconstructed models were checked and assessed by two pancreas surgeons from the pancreatic surgical center of Peking Union Medical College Hospital (PUMCH), and were used in the following training and tests.
Training on Evaluating Pancreatic Tumor Resectability
All residents participating in this study were firstly trained on evaluating the resectability of pancreatic tumors, which was based on the NCCN clinical practice guidelines in Oncology: Pancreatic Adenocarcinoma (2015.V2). Then, a clinical case of pancreatic cancer was taken as a sample for the residents to practice tumor evaluation and surgery planning with conventional continuous axial CT images. The total time was 40 min.
Randomized Grouping
All participants were from the Department of Surgery in PUMCH. Stratified randomization was employed with two factors. Since different postgraduate years (PGYs) or genders may have influence on the remarks, years of training (PGY1, PGY2, PGY3, and PGY4) and genders were both used as stratification factors. Zelen’s algorithm was used by an invited staff from Department of Education without any conflict of interest and signed confidentiality agreement.7 Besides, the invited staff did not participate in the subsequent study.
Self-Directed Learning with 3D Models or 2D CT Images
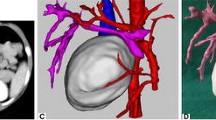
After the basic training, the residents in 3D group were divided into several subgroups to learn the 3D reconstruction model of the sample case on the MVTs for half an hour, and meanwhile the 2D group continued studying this sample case with the axial CT images (Fig. 1).
Imaging Test and Questionnaire
After the theoretical learning and practice with 2D CT or 3D pancreatic models, both groups completed a same test consisting of two pancreatic cases with continuous axial CT images. Fourteen questions in aspects of anatomy, diagnosis, tumor staging, and surgery planning were developed by a group of pancreatic surgeon experts and were contained in each case (Table 1). Each question had one to four points that corresponded to detailed answers. Possible scores for each case ranged from 0 (all incorrect) to 14 (all correct). Each test paper was graded automatically by the test system.
At the end of the test, all residents completed a questionnaire, which was based on questionnaires of the System for the Evaluation of the Teaching Qualities (SETQ) and several previous studies about subjective measures toward simulation-based education.8,9,10 The questionnaire was designed to assess the residents’ attitudes toward the effects of this training on anatomy, imaging, and surgery. All the questions were rated on a 5-point Likert-type scale (Table 2).
Statistical Analysis
The distribution of continuous data was calculated, and the data were described as the mean ± standard deviation (SD). A two-sided unpaired Student’s t test was applied to evaluate the influence of the 3D visualized pancreatic model on the sum scores, and the level of statistical significance was set at a p value of < 0.05. Chi-square test was used to compare categorical variables among the cohorts. Statistical analyses were performed using SPSS (version 23.0 for Mac).
Ethical Approval
Ethical approval was obtained from the Institutional Review Board of the Institute of Basic Medical Sciences, Chinese Academy of Medical Sciences (Project No. 009-2014). All participants completed written informed consent. Study methods were performed in accordance with approved guidelines.
Results
Characteristics of Residents
A total of 88 surgical residents (77 males and 11 females) participated, and there was no prior exposure to the Sectra table. Forty-four residents, consisting of 19 PGY1, 12 PGY2, 9 PGY3, and 4 PGY4 residents, were randomized into the 3D group, and 44 residents, consisting of 20 PGY1, 11 PGY2, 10 PGY3 and 3 PGY4 residents, were grouped into the CT group. In the 3D model group, 25% of the resident had previously assisted in pancreatic surgeries, and 23% in the 2D CT group had the same experience. There were no statistically significant differences in gender (p = 0.747), PGYs (p = 0.967), or previous pancreatic surgery experience (p = 0.803) (Table 3).
Scores of the Test
Overall, the mean scores for questions, in aspects of tumor staging and surgery planning, were significantly higher in the 3D group compared with the 2D group for both cases (case 1: 3D vs. CT, ①Tumor staging: mean difference (MD) = 0.68, p < 0.001. ②Surgery planning: MD = 1.00, p < 0.001. case 2: 3D vs. CT, ①Tumor staging: MD = 0.84, p < 0.001. ②Surgery planning: MD = 0.93, p < 0.001), whereas significant differences were not observed regarding with the scores in the anatomy and diagnosis part (3D vs. CT, case 1: MD = 0.32, p = 0.06. case 2: MD = 0.25, p = 0.14.) (Table 4).
Answers to the Questionnaire
The feedback for the questions (Table 2) related to anatomy indicated that the 3D reconstructed models had great value in improving understanding of complex structures (3D vs. 2D. question 5: MD = 0.52, p < 0.001; question 6: MD = 0.61, p < 0.001). However, both trainings were regarded as being helpful in imaging reasoning and surgery planning (question 7: MD = − 0.16, p = 0.19; question 8: MD = − 0.11, p = 0.34). When asked about their personal attitudes toward these trainings, residents from both groups considered that the learning pattern they just underwent was the best and was efficient in information transfer (question 1: MD = 0.16, p = 0.21; question 2: MD = 0.16, p = 0.20). In addition, residents from the 3D group agreed to allocate more time to the 3D models learning part, while the 2D group thought that time for CT interpretation was enough (question 3: MD = 0.82, p < 0.001). Feedback for question about surgery indicated that training containing 3D models significantly increased residents’ interest in surgery, and residents trained with 3D models highly agreed on introduction of this tool into the surgical resident program (question 4: mean score = 4.75 ± 0.49; question 9: MD = 0.64, p < 0.001.).
Discussion
Surgery is currently the only potential curative method for pancreatic cancer. Because of the anatomical complexities and variations of vessels, the success of the treatment depends on a precise evaluation of the tumor resectability and surgery planning. With the rapid development of digital technologies, 3D visualization system has shown increasing potential value in patient-specific surgeries for its advantages in improving intuition and simplifying complex structures. Application of 3D liver models has revealed their value in precise hepatectomy, in which 3D liver models, based on CT or MR images from clinical patients, facilitated the preoperative planning, intraoperative identification, and resection of liver and vessels.11, 12 Another study, exploring the effects of 3D reconstruction of peripancreatic vascular system on pancreatic surgeries, found that this novel application could apparently reduce surgical trauma and decrease operative time.13 Although the 3D technology has achieved initial success in clinical application, it is still not systematically introduced into the standardized residency training. Thus, we designed this study to explore the value of 3D visualized pancreatic model in tumor evaluation and surgery planning for surgical trainees.
During the training, residents from the 3D group interacted with pancreatic tumor models on the MVTs in the following order: (1) To identify peripancreatic arteries in artery preset. Trainees traced the major peripancreatic arteries in the 360 degree-view reconstructed model and compared the corresponding structures in the 2D CT images on the coronal, axial, and sagittal planes via the crosshair function (Fig. 2). (2) To clarify the spatial relationship between the tumor and adjacent arteries in the artery-organ preset via the crosshair function again. Based on identification of major arteries and tumors previously, trainees inferred the precise location of tumors and their spatial relationship with adjacent arteries in this model. In addition, they simulated surgical procedures by slice cutting in the coronal direction (Fig. 3a). (3) To understand the spatial relationship between the tumor and adjacent arteries and veins in the vein-organ preset (Fig. 3b). It was worth mentioning that the MVT was further explored by some residents, and an air preset was developed to study the spatial relationship among other abdominal vessels and stomach and colon, which reflected its potential value in allowing trainees to be innovative and creative.
In the design of the test, to evaluate surgical trainees’ anatomy-image-surgery knowledge system, the questions were mainly classified into three groups including “anatomy and diagnosis,” “tumor staging,” and “surgery planning” for a better further analysis of the corresponding drill. Both groups used 2D CT images during the test, considering that according to the NCCN guidelines for pancreatic adenocarcinoma, decisions about diagnostic management and resectability rely on the 2D image, that appropriate high-quality imaging studies including pancreatic CT (preferred) or MRI with contrast. 3D image could be as additional reference. Thus, although 3D group were trained with 2D and 3D images, the test was still designed with 2D images of pancreatic CT. Moreover, the learning pattern of CT images could be summarized as the process of “2D→3D→2D,” in which 3D structures primarily formed through cross-sectional 2D CT images in the trainees’ mind, and the following CT interpretation was based on the 3D structures that were previously built. Depending on this, 3D training was given to the surgical residents to improve the spatial learning and accurate understanding of the anatomic relationship between tumors and adjacent tissues, via the real-time corresponding learning between 2D and 3D structures. In addition, designing the tests without 3D models might better assess the effects of 3D training on the reconstruction process of the surgical trainees.
Results of the present study showed that residents in the 2D group had lower scores for both two test cases, which might be an indirect evidence that the visual-spatial ability of surgical residents may not be adequate to interpret 2D to 3D images accurately. Moreover, residents after training with 3D real-time reconstructed models had a better performance in more complex clinical questions, such as those related to tumor staging and surgery planning, which may demonstrate the advantages of 3D virtual models in simplifying complex structures and improving understanding of spatial relationship between tumors and adjacent structures.
In the feedback of questionnaires, from questions 1 and 2, it was interesting to observe that trainees in both 2D group and 3D group considered their training pattern which they have had as the best teaching pattern and efficient information transfer method. A possible explanation might be that both trainings indeed improved participants’ knowledge in anatomy, imaging reasoning, and surgery planning, which could be demonstrated by the high scores of corresponding test questions. However, the significant differences between test scores of tumor staging and surgery planning might provide insight in the effect of 3D models on improving residents’ ability in tumor evaluation and treatment decisions. Another reason for this was that high acceptability toward the conventional CT training among the trainees in the 2D group might be partly related to their lack of knowledge on the effect of 3D virtual technology combined with pancreatic surgery planning training.
The Sectra table is an ergonomic, multi-touch display workstation that lets users simply touch the screen and interact with the 3D models intuitively. During the 3D models training part, residents interacted with 3D models in small groups, and everyone immersed himself or herself in the operation and discussion from beginning to the end. This result was also confirmed by the feedback of the questionnaires, as residents from 3D group agreed to allocate more time to the self-learning part, while feedback from the 2D group was negative. This difference indirectly indicated that the virtual table might add more interest to the process of building the anatomy-image-surgery knowledge system. In addition, feedback of question about training modes showed a preference to the group learning and case-based learning patterns. These suggestions inspired us to develop a more interesting and efficient training mode combining real clinical cases with group-learning patterns in the future.
Regarding the cost, there was limited study about the comparison between traditional and 3D modalities for training purpose. However, in clinical applications, Sean S. Li et al. demonstrated a reduction in the total cost of cases of head and neck reconstruction using computer-aided surgical simulation (CASS) compared with traditional option.14 In our study, the software and equipment costs of Sectra table were approximately $250,000. Considering the time spent from the attendings, a series of cases with key vessels for surgery marked on the Sectra table were designed, with an average of 1–2 h per case, and surgical residents can self-learn in mini groups, without the supervision of the attending physician. The attending physician can also introduce real-life cases into the 3D imaging system for direct face-to-face training without the need for more time to prepare.
Our study has several limitations. First, this is a single-center study in a leading teaching hospital of the country and is biased by center selection. In the future, multi-center study can help improve our current knowledge of the potential value of introduction of 3D technology into pancreatic surgery planning training during different levels of centers and extend its application in other subspecialties. Besides, a systematic training program designed with more training time and including more hepato-pancreato-biliary tumor cases can give us deeper insight into the impact of 3D technology in surgical oncology training, particularly in tumor evaluation and surgery planning. Also, given that there is currently no systematic 3D surgical resident training in our institution, complete data was not collected on this variable, previous 3D training time, which could be considered in the future multi-center study.
Conclusion
This randomized study revealed that the 3D real-time visualization table may have the potential to be a valuable supplemental learning tool in building anatomy-image-surgery knowledge system and thus making surgery planning for surgeon trainees, as it provided a better 3D understanding of the tumor and its surroundings and demonstrated advantages for interacting with cross sectional images. Therefore, it is recommended that this 3D visualization technology, especially with real clinical cases, be systematically introduced into surgical residency for pancreatic surgery training.
References
K. Polireddy, Q. Chen, Cancer of the Pancreas: Molecular Pathways and Current Advancement in Treatment, J Cancer, 7 (2016) 1497–1514.
Blum MG, Powers TW, Sundaresan S. Bronchoscopy simulator effectively prepares junior residents to competently perform basic clinical bronchoscopy. Ann Thorac Surg. 2004;78:287–291.
Yoder D M, Fowler W C, Hey L A. Anatomic eye models as a patient education modality[J]. Ophthalmic Practice, 2001, 19(4):150–156.
Yang X, Hu J, Zhu S, Liang X, Li J, Luo E. Computer-assisted surgical planning and simulation for condylar reconstruction in patients with osteochondroma. Br J Oral Maxillofac Surg. 2011;49:203–208.
Hu A, Wilson T, Ladak H, Haase P, Doyle P, Fung K. Evaluation of a three-dimensional educational computer model of the larynx: Voicing a new direction. J Otolaryngol Head Neck Surg. 2010;39:315–322.
Nicholson DT, Chalk C, Funnell WR, Daniel SJ. Can virtual reality improve anatomy education? A randomised controlled study of a computer-generated three-dimensional anatomical ear model. Med Educ. 2006;40:1081–1087.
Zelen M. A New Design for Randomized Clinical Trials. NEJM. 1979, 300(22), 1242–5.
van der Leeuw R, Lombarts K, Heineman MJ, et al. Systematic evaluation of the teaching qualities of Obstetrics and Gynecology faculty: reliability and validity of the SETQ tools[J]. Plos One, 2011, 6(5):e19142.
Lombarts KM, Bucx MJ, Arah OA. Development of a system for the evaluation of the teaching qualities of anesthesiology faculty[J]. Anesthesiology, 2009, 111(4):709–16.
Seagull F J, Rooney D M. Filling a void: Developing a standard subjective assessment tool for surgical simulation through focused review of current practices[J]. Surgery, 2014, 156(3):718–722.
Mutter D, Soler L, Marescaux J. Recent advances in liver imaging.[J]. Expert Rev Gastroenterol Hepatol, 2010, 4(5):613.
Uchida M. Recent advances in 3D computed tomography techniques for simulation and navigation in hepatobiliary pancreatic surgery[J]. J Hepatobiliary Pancreat Sci, 2014, 21(4):239–245.
Fang CH, Kong D, Wang X, et al. Three-dimensional reconstruction of the peripancreatic vascular system based on computed tomographic angiography images and its clinical application in the surgical management of pancreatic tumors[J]. Pancreas, 2014, 43(3):389–395.
Li SS, Copeland-Halperin LR, Kaminsky AJ, et al. Computer-Aided Surgical Simulation in Head and Neck Reconstruction: A Cost Comparison among Traditional, In-House, and Commercial Options. Journal of reconstructive microsurgery. 2018 Jun;34(05):341–7.
Acknowledgments
The authors appreciate the support from all the surgical residents from PUMCH. This work was partly presented at Pancreas 2018, Innovations in the Care of Pancreatic Cancer, April 26-29, 2018, Maryland, USA.
Funding
This project was supported by grants from funds for Peking Union Medical College Postgraduate Education Reform Program (No. 10023201600101) and funds for Peking Union Medical College Education Reform Program (No. 2015zlgc0126).
Author information
Authors and Affiliations
Contributions
C.L. and J.G. conducted the study, analyzed the results, and wrote the article. H.Z., J.Z., H.Y., G.L., H.L., and H.P. collected the data. Q.L. and Y.Z. designed the trial. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Chen Lin and Junyi Gao are co-first authors
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Lin, C., Gao, J., Zheng, H. et al. Three-Dimensional Visualization Technology Used in Pancreatic Surgery: a Valuable Tool for Surgical Trainees. J Gastrointest Surg 24, 866–873 (2020). https://doi.org/10.1007/s11605-019-04214-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-019-04214-z