Abstract
Objectives
The objective of the present study is to explore the effect of lentivirus-mediated HuR interference on the development and progression of postoperative ileus and the role of HuR in the regulation of the p38/MAPK-activated protein kinase-2 (MK2) signaling pathway during postoperative ileus.
Methods
To establish a mouse model of lentiviral transduction, we first determined the optimum effective titer of lentiviral vectors for transduction of the murine small intestine via the abdominal cavity by using hematoxylin and eosin (HE) staining, immunohistochemistry, detection of GFP messenger RNA (mRNA) and protein, and Western blotting. To investigate the effect of HuR interference on gene expression during postoperative ileus, we established a mouse model of postoperative ileus and used RT-PCR to measure the expression of proinflammatory genes, ELISA to measure the expression of serum inflammatory cytokines, immunohistochemistry to evaluate inflammatory cell infiltration in the small intestine, HE staining of paraffin sections to examine the pathology of the small intestine, and Western blotting to measure HuR expression and identify its role in the regulation of the p38/MK2 inflammatory pathway.
Results
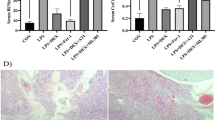
We successfully designed a mouse model of intraperitoneal transduction of HuR-RNAi lentivirus. When HuR gene expression was suppressed in a mouse model of postoperative ileus, the infiltration of inflammatory cells, the expression of proinflammatory genes, and the levels of serum inflammatory cytokines were significantly reduced. This reduction in inflammation correlated with reduced cytoplasmic localization of HuR and reduced activation of MK2.
Conclusions
Within the p38/MK2 signal transduction pathway, HuR may increase the mRNA stability of various inflammatory cytokines, thereby promoting inflammation that causes postoperative ileus. Suppressing the expression of HuR in a postoperative ileus model can effectively suppress the postoperative ileus inflammatory reaction. HuR might serve as a candidate drug target for the prevention and mitigation of postoperative ileus.





Similar content being viewed by others
References
Kalff JC, Buchholz BM, Eskandari MK, et al. Biphasic response to gut manipulation and temporal correlation of cellular infiltrates and muscle dysfunction in rat. Surgery 1999;126:498–509.
Kalff JC, Schraut WH, Simmons RL, et al. Surgical manipulation of the gut elicits an intestinal muscularis inflammatory response resulting in postsurgical ileus. Ann Surg 1998;228:652–63.
Liu X, Wu Chi P, Inhibition of MK2 shows promise for preventing postoperative ileus in mice. Surg Res 2013;185:102–12.
Fan J, Ishmael FT, Fang X, et al. Chemokine transcripts as targets of the RNA-binding protein HuR in human airway epithelium. J Immunol 2011, 186:2482–94.
Rhee WJ, Ni CW, Zheng Z, et al. HuR regulates the expression of stress-sensitive genes and mediated inflammatory response in human umbilical vein endothelial cells. Proc Natl Acad Sci USA 2010;107:6858–63.
Espel E. The role of the AU-rich elements of mRNAs in controlling translation. Semin Cell Dev Biol 2005;16:59–67.
Dai W, Zhang G, Makeyev EV. RNA-binding protein HuR autoregulates its expression by promoting alternative polyadenylation site usage. Nucleic Acids Res 2012;40:787–800.
Xu J, Shi J, Lu X, Su X, Shi Y. MK2 and HuR expression in lung tissues in mice with acute lung injury. Chinese Critical Care Medicine 2013;33:160–3.
Kotlyarov A, Neininger A, Schubert C, et al. MAPKAP kinase 2 is essential for LPS-induced TNF-alpha biosynthesis. Nat Cell Biol 1999;1:94–7.
Neininger A, Kontoyiannis D, Koflyarov A, et al. MK2 targets AU-rich elements and regulates biosynthesis of tumor necrosis factor and interleukin-6 independently at different post-transcriptional levels. J Biol Chem 2002;277:3065–8.
Schnyder-Candrian S, Quesniaux VF, Di Padova F, et al. Dual effects of p38 MAPK on TNF-dependent bronchoconstriction and TNF-dependent neutrophil recruitment in lipopolysaccharide-induced acute respiratory distress syndrome. J Immunol 2005;175:262–9.
Wehner S, Straesser S, Vilz T, et al. Inhibition of p38 mitogen-activated protein kinase pathway as prophylaxis of postoperative ileus in mice. Gastroenterology 2009;136:619–29.
Tietz AB, Malo A, Diebold J, et al. Gene deletion of MK2 inhibits TNF-alpha and IL-6 and protects against cerulein-induced pancreatitis. Am J Physiol Gastrointest Liver Physiol 2006;290:G1298-306.
Ross J. mRNA stability in mammalian cells. Microbiol Rev 1995;59:423–50.
Brennan CM, Steitz JA. HuR and mRNA stability. Cell Mol Life Sci 2001;58:266–77.
Farooo F, Balabanian S, Liu X, et al. p38 Mitogen -activated protein kinase stabilizes SMN mRNA through RNA binding protein HuR. Hum Mol Genet, 2009;18:4035–45.
Schalijo K. Tristetraprolin is required for full anti-inflammatory response of murine macrophages to IL-10. J Immunol 2009;183:1197–206.
Duraisamy S, Baijpai M, Bughani U, et al. MK2: a novel molecular target for anti-inflammatory therapy. Expert Opin Ther Targets 2008;12:921–36.
Ming XF, Stoecklin G, Lu M, et al. Parallel and independent regulation of interleukin-3 mRNA turnover by phosphatidylinositol 3-kinase and p38 mitogen-activated protein kinase. Mol Cell Biol 2001;21:5778–89.
Guarantor of the Article
Chi Pan accepts full responsibility for the conduct of the study, has had access to the data, and has control of the decision to publish.
Specific Author Contributions
Ye Dao Xiong and Chi Pan conceived the study, collected and analyzed the data, and drafted the manuscript. Lu Xing Rong critically reviewed and revised the manuscript. All authors read and approved the final manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Financial Support
This study was supported by the National Key Clinical Specialty Discipline Construction Program of China (No. 2015-8147277) and the Key Project of Science and Technology Plan of Fujian Province, China (No. 2011J01172 and 2012J01349).
Conflict of Interest
The authors declare that they have no conflicts of interest.
Additional information
Study Highlights
1. What is the current knowledge?
• Currently, there is no effective method to prevent postoperative ileus.
• Inflammatory response arising 3–4 h postoperation is the leading cause of postoperative ileus.
• There is no targeted therapy that prevents postoperative inflammatory responses.
2. What is new here?
• HuR promotes postoperative inflammation by stabilizing mRNAs of inflammatory cytokines.
• Blocking HuR expression ameliorates inflammation in a mouse model of postoperative ileus.
• HuR may provide a potential therapeutic target to prevent and reduce postoperative ileus.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 22 kb)
Supplementary Figure 1
(GIF 242 kb)
Rights and permissions
About this article
Cite this article
Xiong, Y.D., Rong, L.X. & Pan, C. Regulation of Postoperative Ileus by Lentivirus-Mediated HuR RNA Interference via the p38/MK2 Signaling Pathway. J Gastrointest Surg 21, 389–397 (2017). https://doi.org/10.1007/s11605-016-3303-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-016-3303-z