Abstract
Background
Surgery for early esophageal carcinoma has been challenged by less invasive endoscopic approaches. Selecting patients in need for surgical intervention according to their risk of lymphatic spread is mandatory.
Objective
The aim of this study was to evaluate risk factors for lymphatic metastasis formation in T1b esophageal carcinomas.
Methods
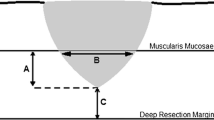
Histopathological specimens following surgical resection for T1b esophageal carcinomas were reevaluated for overall submucosal layer thickness, depth of submucosal tumor infiltration, tumor length as well as lymphatic and vascular infiltration. Depth of tumor infiltration to overall submucosal thickness was divided in thirds (SM1, SM2, and SM3) and factors influencing lymphatic metastasis formation were assessed.
Results
A total of 67 patients with pT1b tumors were analyzed, including 36 adenocarcinomas (53.7 %) and 31 squamous cell carcinomas (46.3 %). Lymph node involvement was seen in 22.4 % (15/67) patients without significant differences between SM1 3/11 (27.3 %), SM2, 4/18 (22.2 %), and SM3 (8/38) (21.8 %) (p = 0.909) carcinomas. On binomial log-regression models, only lymphangioinvasion and tumor length >2 cm was significantly associated with lymph node involvement.
Conclusion
As depth of submucosal tumor infiltration did not correlate with the formation of lymph node metastases and in regard of the risk of lymphatic spread in these cases, surgical resection is warranted in pT1b carcinomas.



Similar content being viewed by others
References
van Hagen P, Hulshof MC, van Lanschot JJ, Steyerberg EW, van Berge Henegouwen MI, Wijnhoven BP, Richel DJ, Nieuwenhuijzen GA, Hospers GA, Bonenkamp JJ, Cuesta MA, Blaisse RJ, Busch OR, ten Kate FJ, Creemers GJ, Punt CJ, Plukker JT, Verheul HM, Spillenaar Bilgen EJ, van Dekken H, van der Sangen MJ, Rozema T, Biermann K, Beukema JC, Piet AH, van Rij CM, Reinders JG, Tilanus HW, van der Gaast A. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 2012; 366: 2074–2084
Ell C, May A, Pech O, Gossner L, Guenter E, Behrens A, Nachbar L, Huijsmans J, Vieth M, Stolte M. Curative endoscopic resection of early esophageal adenocarcinomas (Barrett's cancer). Gastrointest Endosc 2007; 65: 3–10
Pech O, Behrens A, May A, Nachbar L, Gossner L, Rabenstein T, Manner H, Guenter E, Huijsmans J, Vieth M, Stolte M, Ell C. Long-term results and risk factor analysis for recurrence after curative endoscopic therapy in 349 patients with high-grade intraepithelial neoplasia and mucosal adenocarcinoma in Barrett's oesophagus. Gut 2008; 57: 1200–1206
Boshier PR, Anderson O, Hanna GB. Transthoracic versus transhiatal esophagectomy for the treatment of esophagogastric cancer: a meta-analysis. Ann Surg 2011; 254: 894–906
Holscher AH, Bollschweiler E, Bumm R, Bartels H, Hofler H, Siewert JR. Prognostic factors of resected adenocarcinoma of the esophagus. Surgery 1995; 118: 845–855
Bogoevski D, Bockhorn M, Koenig A, Reeh M, von Loga K, Sauter G, Rosch T, Izbicki JR. How radical should surgery be for early esophageal cancer? World J Surg 2011; 35: 1311–1320
Sobin LH, Gospodarowicz MK, Wittekind C TNM Classification of malignant tumours, Wiley, 2011
Rice TW, Zuccaro G, Jr., Adelstein DJ, Rybicki LA, Blackstone EH, Goldblum JR. Esophageal carcinoma: depth of tumor invasion is predictive of regional lymph node status. Ann Thorac Surg 1998; 65: 787–792
Kutup A, Link BC, Schurr PG, Strate T, Kaifi JT, Bubenheim M, Seewald S, Yekebas EF, Soehendra N, Izbicki JR. Quality control of endoscopic ultrasound in preoperative staging of esophageal cancer. Endoscopy 2007; 39: 715–719
Nigro JJ, Hagen JA, DeMeester TR, DeMeester SR, Peters JH, Oberg S, Theisen J, Kiyabu M, Crookes PF, Bremner CG. Prevalence and location of nodal metastases in distal esophageal adenocarcinoma confined to the wall: implications for therapy. J Thorac Cardiovasc Surg 1999; 117: 16–23; discussion 23–15
van Sandick JW, van Lanschot JJ, ten Kate FJ, Offerhaus GJ, Fockens P, Tytgat GN, Obertop H. Pathology of early invasive adenocarcinoma of the esophagus or esophagogastric junction: implications for therapeutic decision making. Cancer 2000; 88: 2429–2437
Stein HJ, Feith M, Mueller J, Werner M, Siewert JR. Limited resection for early adenocarcinoma in Barrett's esophagus. Ann Surg 2000; 232: 733–742
Liu L, Hofstetter WL, Rashid A, Swisher SG, Correa AM, Ajani JA, Hamilton SR, Wu TT. Significance of the depth of tumor invasion and lymph node metastasis in superficially invasive (T1) esophageal adenocarcinoma. Am J Surg Pathol 2005; 29: 1079–1085
Oh DS, Hagen JA, Chandrasoma PT, Dunst CM, Demeester SR, Alavi M, Bremner CG, Lipham J, Rizzetto C, Cote R, Demeester TR. Clinical biology and surgical therapy of intramucosal adenocarcinoma of the esophagus. J Am Coll Surg 2006; 203: 152–161
Altorki NK, Lee PC, Liss Y, Meherally D, Korst RJ, Christos P, Mazumdar M, Port JL. Multifocal neoplasia and nodal metastases in T1 esophageal carcinoma: implications for endoscopic treatment. Ann Surg 2008; 247: 434–439
Pennathur A, Farkas A, Krasinskas AM, Ferson PF, Gooding WE, Gibson MK, Schuchert MJ, Landreneau RJ, Luketich JD. Esophagectomy for T1 esophageal cancer: outcomes in 100 patients and implications for endoscopic therapy. Ann Thorac Surg 2009; 87: 1048–1054; discussion 1054–1045
Sepesi B, Watson TJ, Zhou D, Polomsky M, Litle VR, Jones CE, Raymond DP, Hu R, Qiu X, Peters JH. Are endoscopic therapies appropriate for superficial submucosal esophageal adenocarcinoma? An analysis of esophagectomy specimens. J Am Coll Surg 2010; 210: 418–427
Griffin SM, Burt AD, Jennings NA. Lymph node metastasis in early esophageal adenocarcinoma. Ann Surg 2011; 254: 731–736; discussion 736–737
Dunbar KB, Spechler SJ. The risk of lymph-node metastases in patients with high-grade dysplasia or intramucosal carcinoma in Barrett's esophagus: a systematic review. Am J Gastroenterol 2012; 107: 850–862; quiz 863
Ancona E, Rampado S, Cassaro M, Battaglia G, Ruol A, Castoro C, Portale G, Cavallin F, Rugge M. Prediction of lymph node status in superficial esophageal carcinoma. Ann Surg Oncol 2008; 15: 3278–3288
Buskens CJ, Westerterp M, Lagarde SM, Bergman JJ, ten Kate FJ, van Lanschot JJ. Prediction of appropriateness of local endoscopic treatment for high-grade dysplasia and early adenocarcinoma by EUS and histopathologic features. Gastrointest Endosc 2004; 60: 703–710
Westerterp M, Koppert LB, Buskens CJ, Tilanus HW, ten Kate FJ, Bergman JJ, Siersema PD, van Dekken H, van Lanschot JJ. Outcome of surgical treatment for early adenocarcinoma of the esophagus or gastro-esophageal junction. Virchows Arch 2005; 446: 497–504
Bollschweiler E, Baldus SE, Schroder W, Prenzel K, Gutschow C, Schneider PM, Holscher AH. High rate of lymph-node metastasis in submucosal esophageal squamous-cell carcinomas and adenocarcinomas. Endoscopy 2006; 38: 149–156
Bolton WD, Hofstetter WL, Francis AM, Correa AM, Ajani JA, Bhutani MS, Erasmus J, Komaki R, Maru DM, Mehran RJ, Rice DC, Roth JA, Vaporciyan AA, Walsh GL, Swisher SG. Impact of tumor length on long-term survival of pT1 esophageal adenocarcinoma. J Thorac Cardiovasc Surg 2009; 138: 831–836
Yendamuri S, Swisher SG, Correa AM, Hofstetter W, Ajani JA, Francis A, Maru D, Mehran RJ, Rice DC, Roth JA, Walsh GL, Vaporciyan AA. Esophageal tumor length is independently associated with long-term survival. Cancer 2009; 115: 508–516
Wang BY, Goan YG, Hsu PK, Hsu WH, Wu YC. Tumor length as a prognostic factor in esophageal squamous cell carcinoma. Ann Thorac Surg 2011; 91: 887–893
Gockel I, Domeyer M, Sgourakis GG, Schimanski CC, Moehler M, Kirkpatrick CJ, Lang H, Junginger T, Hansen T. Prediction model of lymph node metastasis in superficial esophageal adenocarcinoma and squamous cell cancer including D2-40 immunostaining. J Surg Oncol 2009; 100: 191–198
Dicken BJ, Graham K, Hamilton SM, Andrews S, Lai R, Listgarten J, Jhangri GS, Saunders LD, Damaraju S, Cass C. Lymphovascular invasion is associated with poor survival in gastric cancer: an application of gene-expression and tissue array techniques. Ann Surg 2006; 243: 64–73
Ishii M, Ota M, Saito S, Kinugasa Y, Akamoto S, Ito I. Lymphatic vessel invasion detected by monoclonal antibody D2-40 as a predictor of lymph node metastasis in T1 colorectal cancer. Int J Colorectal Dis 2009; 24: 1069–1074
Arnaout-Alkarain A, Kahn HJ, Narod SA, Sun PA, Marks AN. Significance of lymph vessel invasion identified by the endothelial lymphatic marker D2-40 in node negative breast cancer. Mod Pathol 2007; 20: 183–191
Conflict of Interest
The authors deny any potential or real conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
This study was not supported by any grants.
Discussion
Dr. Steven R. DeMeester (Los Angeles, CA): I congratulate the authors on a nice presentation and important study and continued contribution to this important field in esophageal cancer. I also commend you for reevaluation of the specimens pathologically rather than just relying on the pathology reports since over the years of course the staging system has changed and there is a renewed focus on the differentiation between T1A and T1B tumors.
In that respect, can you tell me how often the pathology changed on your reevaluation, particularly in these older specimens, from what the reports said to what you actually found on your reevaluation? Within the T1B, how often did you do your reevaluation, was it in fact either deeper or more superficial?
Secondly, in the node-negative patients did you consider humanistic chemistry for micrometastasis since that may actually lead to a higher prevalence of nodal disease than what you showed with H&E and routine processing.
And lastly, you showed no overall difference in the prevalence of lymph node metastasis based on the depth of semicosal invasion, but was there a low-risk group such as Elle and colleagues suggested might exist in small SM1 tumors with no lymphovascular invasion and well-differentiated histology? Did you look specifically at tumors that had all of those or lacked any of those high-risk features and what was the prevalence of no metastasis in that specific group?
Closing Discussant
Dr. Dean Bogoevski: Thank you, Dr. DeMeester for your insightful comments and remarks. It was a challenge for us too, to reevaluate the pathological specimens. Especially in patients operated before 1997 and the 5th edition of the TNM Classification of the UICC, since in the old pathological reports there was no subdivision in pT1a and pT1b. Also, as you were able to see in the presentation, the oldest pathological specimens were already 20 years old, and in some cases even an experienced pathologist had problems to reevaluate them. Therefore, we had to exclude three patients from our cohort since the pathological specimens were no more usable for reevaluation. Concerning your question about the changing of the pathology, in 11 patients the pathological reports were changed after the reevaluation, but all of them within the pT1b, 3 patients from sm1 into sm2, 6 patients from sm2 into sm3, and 1 patient from sm3 into sm2.
Regarding the micrometastasis, we were also curious to find it out, but the time we had to prepare the manuscript did not allow us to finish the immunohistochemical staining. We started with the staining recently and we will report the results in the near future. However, I would like to quote the manuscript from our group published in Annals of Surgical Oncology in 2009 (Koenig et al.) in which we were able to show that the detection of nodal microinvolvement in pN0 patients with esophageal carcinoma worsened the 5-year survival rate from 75 to 30 %.
On your last question, I have to answer with yes, since we had in fact three patients with SCC pT1b, SM1, without lymphovascular invasion and well-differentiated histology that did not have lymph node metastasis. On the other hand, we also had 3 patients with SCC sm2, without lymphovascular invasion and well-differentiated histology that had lymph node metastasis. And the accuracy of the staging into thirds after ESD, as shown in the presentation, was poor. Therefore, factors like grading and lymphovascular invasion, although playing a pivotal role, still cannot give certainty about the nodal involvement in these patients.
Michael F. Nentwich and Katharina von Loga contributed equally to the presented work and therefore share first authorship.
Rights and permissions
About this article
Cite this article
Nentwich, M.F., von Loga, K., Reeh, M. et al. Depth of Submucosal Tumor Infiltration and its Relevance in Lymphatic Metastasis Formation for T1b Squamous Cell and Adenocarcinomas of the Esophagus. J Gastrointest Surg 18, 242–249 (2014). https://doi.org/10.1007/s11605-013-2367-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-013-2367-2