Abstract
Introduction
Various techniques have been described to achieve definitive resolution of complex acute pancreatitis associated pseudocysts (PACs). Many of these strategies, inclusive of open, minimally invasive, and radiological procedures, are hampered by high recurrence or failed resolution, particularly for PAC near the pancreatic head. The present series describes a multimodal strategy combining a minilaparotomy for anterior gastrostomy for the creation of a stapled posterior cystogastrostomy, placement of an 8F secured silastic tube for intentional formation of a cystogastric fistula tract in combination with gastric drainage, and postduodenal enteral alimentation.
Materials and Methods
Using a prospectively maintained hepatobiliary database, patients with complex PAC undergoing the above procedures were identified. PAC location, postoperative length of stay (LOS), and time to start enteral feeding were identified. PAC were assessed by computed tomography (CT) scan prior to operation, 1 month after drainage, and patients with PAC resolution were started on oral diet, with the fistula silastic tube kept in place for an additional month.
Results
Over the interval 2003 to 2008, 19 patients were managed with the stated strategy. PACs were located at the pancreatic body/tail in 12 patients, and 7 patients had PAC at the level of the pancreatic head/neck area. In this cohort, prior to surgical drainage, 17/19 patients had undergone failed endoscopic retrograde cholangiopancreatography (ERCP) with decompressive stent placement and 13/19 had a failed percutaneous PAC drainage. There was no perioperative mortality after open surgical drainage. All patients started on jejunal tube feeding 24 h after surgical procedure. Median postoperative LOS was 7 days (4–13). At 1 month, 16/19 (84%) of patients showed complete resolution of the PAC on CT scan and were started on oral diet; 3/19 required additional month for complete resolution. After a mean follow-up of 31 months, there was no PAC recurrences in any of these patients demonstrated on follow-up.
Conclusion
The described strategy is safe, efficient, and allows early restoration of enteral feeding with early hospital discharge. High resolution rates and absence of PAC recurrences in this series supports this approach for complex PAC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acute pancreatitis-associated pseudocyst (PAC) is a collection of serous fluid that may contain pancreatic juice as a consequence of inflammatory pancreatitis or ductal leakage that complicates about 10% of acute pancreatitis.1 Although a watchful expectancy policy can result in resolution for 8% to 84% of uncomplicated PACs,2,3 complex PAC including those >5 cm, compressing on surrounding structures resulting in gastric outlet or biliary obstruction and infected PAC, require drainage.3,4
Classical surgical drainage with either direct or Roux limb drainage though the gold standard carries substantial morbidity and mortality of 25% and 5%, respectively.5 Less invasive approaches including laparoscopic internal drainage6–9 and endoscopic10–12 and percutaneous drainage13–15 report success in selected groups of patents. However, most series of laparoscopic drainage lack long-term follow-up8 and report recurrence rates as high as 8%.7 For endoscopic drainage, even though complete early resolution is achieved in 81% to 92%, the recurrence rate is as high as 23% with morbidity of 17%.10–12 Comparable results are also obtained with percutaneous drainage with reported resolution rate of 84%, recurrence rate of 7%, and complication rate of 18%.13
Nowadays, the better understanding of the pathophysiology of PAC16,17 enables the selection of optimal candidates for minimally invasive approaches, with the aim of reduced treatment failure and recurrence. For patients who have failed nonsurgical treatment (endoscopic, percutaneous) and those with PAC directly communicating to the pancreatic duct, surgical management is indicated.18
Herein reported is a consecutive series of patients employing a multimodal strategy applied to 19 patients with complex PAC transferred to our tertiary care facility after failure of ERCP and or percutaneous drainage. This open minilaparotomy procedure combines open anterior gastrostomy for the creation of a stapled posterior cystogastrostomy, placement of an 8F secured silastic tube for intentional formation of a cystogastric fistula tract in combination with gastric drainage, and postduodenal enteral alimentation.
Materials and Methods
Using a prospectively maintained hepatobiliary database, patients with complex PAC who had failed or were not candidates for endoscopic or percutaneous drainage, with identified pancreatic duct communication into the PAC and who underwent the combined surgical procedure as described, were identified (Table 1). Over a 5-year period (2003–2008), 19 patients (12 men/7 women) presented with complex PAC meeting the above criteria. The patients' median age was 51 years (35–75), and the etiology of pancreatitis was alcoholic in eight patients, biliary in seven, hypercholesterolemia in two, and ERCP induced in two. Twelve out of the 19 patients had PAC at the level of the pancreatic body and tail, while in the remaining 7 patients, the PAC was located at the pancreatic head–neck area. All patients were transferred to our service from other facilities after treatment failure, inclusive of 17 ERCP with decompressive stent placement and/or transgastric drainage and 13 percutaneous catheter placements. The interval elapsed between the diagnosis of the PAC, and the transfer to our service was <3 months in 12 patients and >3 months in 7 patients.
Upon transfer, 14/19 patients were on Nil per os (NPO) status, and nutritional repletion was by parenteral alimentation, three patients were on liquid diet, and the last two patients, though on regular diet, experienced intermittent oral intolerance. Fifteen patients were receiving somatostatin analogue, and 17 patients were receiving opioids for pain control. Fourteen patients were febrile with positive blood culture and were started upon admission on broad-spectrum intravenous β-lactam antibiotic as well as antifungal coverage as per protocol. Twelve patients had pancreatic ascites, and one patient had developed venous thromboembolic disease with pulmonary embolism complicated by gastrointestinal bleeding upon anticoagulation. Ten patients had undergone endoscopic placement of a nasojejunal feeding tube; none of which were being utilized for enteral alimentation. Similarly, ten patients were transferred with a nasogastric tube above the gastric outlet obstruction resulting from PAC compression.
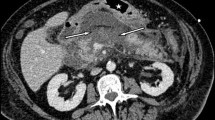
On admission to our institution, all patients had an abdominal computed tomography (CT) scan with Intravenous (IV) contrast to assess the morphology of the PAC and define the relationship between the posterior gastric wall and the splenic artery (Fig. 1). The average PAC size was 12 cm (6–23 cm). The average time elapsed between admission to our service, and the open cystogastrostomy procedure was 19 days (5–27 days). Before surgery, all patients were afebrile and electrolyte abnormalities were corrected.
Technique
For the surgical procedure, patients were placed in supine position under general endotracheal anesthesia; a left subcostal incision was made. Using a Bookwalter retractor, the abdominal cavity was explored. An anterior longitudinal gastrostomy was made at the widest point of the stomach body at the level of the incisura extending down to the antrum (Fig. 2). A fine needle was introduced through the posterior wall of the stomach into the retroperitoneal fluid collection confirming its location and its direct relationship to the posterior gastric wall. The retroperitoneal space was entered through a longitudinal incision on the posterior gastric wall over the fine needle using electrocautery (Fig. 3).
Cystogastrostomy was completed to a total length of about 5 cm using one of two techniques: On the first seven patients, we used electrocautery with imbrication of the gastric–cyst edge with a running 2/0 monofilament nonabsorbable stitch. On the last 12 patients, the cystogastrostomy was completed using an endomechanical stapling device with 3.5-mm staples. Next, the posterior gastric wall was stitched on either site of the cystogastrostomy with 2/0 silk and brought to the wound to facilitate exposure. Through the cystogastrostomy, a pancreatic necrosectomy was performed. A Moss type gastric/jejunal tube was then brought through the anterior abdominal wall and then the anterior gastric wall superior to the anterior gastrostomy in Stamm fashion. Attention was placed to ensure the gastric component (balloon) was within the lumen and the jejunal tube component was placed under direct vision through the pylorus and guided to the proximal jejunum. Next, through a separate stab incision on the anterior gastric wall, an 8F round silastic tube was placed through the anterior gastric wall and placed into the cystogastrostomy to externally drain the retroperitoneal space assuring continuous drainage of the communicating pancreatic ductal leak into the pseudocyst collection.
Both the Moss and the silastic tube were secured to the gastric wall (Stamm) and the skin. Next, the gastrostomy was closed in two layers using a running 3/0 monofilament absorbable stitch first, followed by interrupted 2/0 Lembert stitches. The abdominal wall was then closed in a regular fashion.
Results
All patients but two were extubated on the operating table (15/17). As per protocol, all patients were taken postoperatively to the intensive care unit (ICU) where the two patients initially requiring ventilatory support were extubated by postoperative day 2. Nasogastric tubes were removed from all patients immediately after surgery. Jejunal alimentation was begun in 17/17 patients 24 h after surgery. Fifteen patients were transferred to a regular surgical ward from the ICU after 24 h, while four patients required extra ICU care for respiratory failure (n = 2), intravenous cardiac medication (n = 1), and postoperative arrhythmia (n = 1).
Jejunal tube feeding was tolerated by all patients and was advanced to goal (as per nutrition team) within average of 3 days from the surgery. Both the gastric tube and transgastric retroperitoneal tube were drained to gravity. Prior to hospital discharge, all patients were advanced to water-only PO intake with the gastric/retroperitoneal tubes draining to gravity.
In this series, there was no perioperative mortality. Postoperative morbidities were graded on a scale of 1 to 519,20 (Table 2). Accordingly, two patients had a grade 1 complication as superficial wound infection that required bedside drainage. Two patients had grade 2 complications: postoperative pneumonia (n = 1) and urinary tract infection (n = 1). One patient had grade 3 complication; this patient had a complicated preoperative course marked with pulmonary embolism and deep venous thrombosis, and postoperatively required ICU care for ventilator support and developed pneumonia managed with antibiotic therapy. This same patient was readmitted approximately 3 weeks after discharge with postpneumonic empyema and required thoracotomy with decortication. There were no grade 4 or 5 complications in our series. The median LOS was 7 days (4–13 days).
One month after the surgical drainage, all patients had a CT scan to assess the PAC. In 16/19 patients (84%), the CT scan showed complete resolution of the PAC (Fig. 4). These patients were allowed a regular low-fat diet with the gastrostomy limb of the Moss tube capped for 1 month before its removal. The transgastric retroperitoneal drain was left in place for total a period of 3 months. Patients were advised to refrain entirely from alcohol consumption and follow a low-fat diet.
In the three patients whom the initial 30-day CT scan did not show complete PAC resolution, an additional month of water-only per oral intake with goal jejunal feeding was continued. Subsequent to a repeat CT, with demonstrated PAC resolution, the diet was advanced as previously described.
Clinical follow-up beyond the 6-month visit consisted of an annual visit with either CT scan or magnetic resonance imaging for the first year visit; subsequent need for imaging is symptom driven. Over a mean follow-up period of 31 months (range, 6–60 months), none of the 19 patients in this series developed PAC recurrence. Moreover, radiological evaluation at 1 year after the cystogastrostomy has demonstrated the persistence of the intended fistula tract between the stomach and the retroperitoneum, which was created by the retroperitoneal drainage catheter (Fig. 5).
Discussion
This modest series reports a successful treatment strategy for a complex albeit uncommon problem; patients with pancreatitis associated pseudocyst (PAC) with a pancreatic duct to cyst fistula, which have been refractory to endoscopic or percutaneous treatment. As all patients were referred to our service with the diagnosis of PAC and failed prior treatment, preoperative ERCP results were not included in this report. The vast majority of these patients had a demonstrated direct communication between the PAC and the main pancreatic duct (type VII according to Nealon classification)17 or type II and III according to D'Egidio classification16 likely explaining the failure nonsurgical drainage.
In this series, 19 patients with unrelieved PAC for a relative long period (7/19 patients presented after >3 months from their diagnosis) were successfully treated surgically with a median postoperative LOS of 1 week. Enteral feeding was started early, during the first 24 h after surgery through the combined gastrostomy–jejunostomy tube. Three months after the surgical procedure, 84% of the patients were tolerating regular diet with no drains. This approach was safely performed without perioperative mortality or permanent organ failure in 19 patients including 14 with document preoperative sepsis, and only five patients (26%) had grade 1 to 3 morbidities.
The authors recognize that penetration of antibiotics into pancreatic necroses is minimal and, even then, limited to a particular few. Preoperatively, patients were resuscitated and treated with a combination of broad-spectrum antibiotics (imipenem or ciprofloxacin) in combination with metronidazole, with empiric antifungal coverage. Nutritional support and repletion were initiated and continued immediately postoperatively. It is almost a certainty that there is a relationship between the contaminated or infected pancreas necrosis and the postoperative infection (n = 5).
The described surgical approach was instituted by the senior author, in response to the needs of a select population transferred into a tertiary referral center; in which nonoperative treatment strategies had failed. The expectation from the perspective of an experienced hepatopancreatobiliary (HPB) team would be that for patients with demonstrated pancreatic duct to pseudocyst fistula; percutaneous drainage alone will be insufficient and often fail. While modern endoscopic techniques combining pancreatic duct stent drainage along with transgastric endoscopic pseudocyst drainage have reported improved outcomes over single modality approaches for these specific patients, the expertise for these procedures is still not widely available.
Several clinical lessons were learned through the described experience. First, it is important to have current imaging (CT), preoperatively, to define the direct relationship between the PAC and the posterior gastric wall, as well as to define the course of splenic artery within the PAC. Although not identified in any of these 19 patients, it is imperative to preoperatively exclude the presence of the splenic artery immediately adjacent to the gastric wall, at the level of the planned cystogastrostomy, as failure to recognize this would likely lead to massive intraoperative bleeding.
Next, although the PAC location is usually readily identified as a bulging through the posterior gastric wall upon opening the anterior gastric wall, it is recommended to use a fine needle to aspirate and confirm the location at which to begin the posterior gastrostomy. This technique assures the identification of a fluid containing area rather than debris and necrosis, thus, facilitating the creation of the cystogastrostomy.
In the beginning of this series, electrocautery was used to create the cystogastrostomy, and a running monofilament nonabsorbable stitch was used to imbricate its edges (n = 7); in our opinion, this approach was time consuming and frankly physically uncomfortable due to the limitations of the gastrostomy and limited abdominal incision. Consequently, for the second group of patients (n = 12), an endomechanical stapler was used to complete the cystogastrostomy, for reasons only of efficiency and technical ease.
Previous clinical reports have described the technique of surgical pancreatic necrosectomy and emphasized the importance of postoperative drainage to assure complete resolution. Thus, once necrosectomy was completed, the surgeon still depended on the continued support of interventional radiology through regular exchange of large-bore pancreatic drains, particularly in the presence of known or suspected pancreatic duct leak.21 The herein described technique assures necrotic tissue drainage, a route for decompression and enteral alimentation, as well as a long-term mechanism for continued internal drainage performed as a single surgical procedure, and furthermore avoided the inconvenience of external drainage catheters.
The cornerstone concept in this treatment strategy is the silastic cystogastric tube for retroperitoneal drainage. This drain allows the direct communication between the gastric lumen and retroperitoneal compartments and prevents premature closure of the surgical cystogastrostomy. By keeping this tube in place for 3 months, there is an intentionally induced epithelialization of the cystogastrostomy to assure continued drainage, thus, in our opinion, explaining the absence of PAC recurrence in this series. The evidence to support this concept is that the imaging demonstrated persistence of this fistulous communication in the follow-up radiological studies.
As a final technical note, after discharge, when patients were allowed to start on a regular diet with a capped gastric tube, we recommend intermittent uncapping and gravity drainage of the gastric tube to assure low residual gastric content, before permanently removing the tube.
The described combined technique of wide cystogastrostomy and a transgastric retroperitoneal drainage tube was associated with complete resolution of all complex pancreatic pseudocysts without recurrence in this series. The wide cystogastrostomy promotes adequate internal drainage of the heterogeneous pancreatic pseudocyst contents, resulting in drainage and complete resolution compared to other less invasive techniques. However, this anastomosis (cystogastrostomy) will ultimately close. In these selected cases, all of these patients had previously failed conventional nonoperative means. In the clinical scenario of suspicion or proven pancreatic duct leak into the pseudocyst, the early closure of the cystogastrostomy and/or the continued leakage of pancreatic fluid will result in the reaccumulation of trapped fluid, namely, pancreatic pseudocyst recurrence. The creation of a retroperitoneal-to-gastric drainage fistulous tract, as documented by radiological studies, has been demonstrated after 1 year to maintain adequate decompression and has precluded recurrence.
A limitation of this series is the lack of detailed pretransfer history. Consequently, it is difficult to define the exact onset of acute pancreatitis. However, the interval between the documented pancreatic pseudocyst and the transfer to our facility was <3 months in 12 patients and >3 months for 7 patients.
Another limitation is that the study was not constructed to compare this approach to other previously described strategies but rather to report the efficacy and safety of this treatment strategy when others fail. The authors acknowledge the selection bias in this report, as patients included in this study represent only patients that had already failed prior attempted treatment by various nonsurgical modalities; consequently, it would be inaccurate to advocate that the described approach should be a universal procedure for all pancreatic pseudocysts. On the other hand, we advocate that for patients with complex PAC, when other approaches fail to obtain or maintain PAC resolution, a surgical cystogastrostomy with continuous internal drainage of the retroperitoneum is safe and efficient and should be kept in the surgical armamentarium of options for these complex patients.
In the field of HPB surgery, in particular, to the care of patients with pancreatitis in all of its forms, we have fortunately come to a general agreement that for these patients, institutional or service line teams working in a coordinated fashion achieve the best outcomes. Within this manuscript, there is likely an understated importance of the nutrition support team, physical therapy, and wound care team's contributions to the comprehensive care of these complex patients; the surgical and critical care aspects of management, while important, require a multimodal group of health care practitioners to achieve the best possible outcomes.
References
O'Malley VP, Cannon JP, Postier RG: Pancreatic pseudocysts: cause, therapy, and results. Am J Surg 1985; 150(6): 680–2.
Warshaw AL, Rattner DW: Timing of surgical drainage for pancreatic pseudocyst. Clinical and chemical criteria. Ann Surg 1985; 202(6): 720–4.
Chen KH, Hsu HM, Hsu GC, Chen TW, Hsieh CB, Liu YC, et al.: Therapeutic strategies for pancreatic pseudocysts: experience in Taiwan. Hepatogastroenterology 2008; 55(85): 1470–4.
Aghdassi AA, Mayerle J, Kraft M, Sielenkamper AW, Heidecke CD, Lerch MM: Pancreatic pseudocysts—when and how to treat? HPB (Oxford) 2006; 8(6): 432–41.
Bergman S, Melvin WS: Operative and nonoperative management of pancreatic pseudocysts. Surg Clin North Am. 2007; 87(6): 1447–60, ix.
Teixeira J, Gibbs KE, Vaimakis S, Rezayat C: Laparoscopic Roux-en-Y pancreatic cyst-jejunostomy. Surg Endosc 2003; 17(12): 1910–3.
Zhou ZG, Zheng YC, Shu Y, Hu WM, Tian BL, Li QS, et al.: Laparoscopic management of severe acute pancreatitis. Pancreas 2003; 27(3): e46–50.
Bhattacharya D, Ammori BJ: Minimally invasive approaches to the management of pancreatic pseudocysts: review of the literature. Surg Laparosc Endosc Percutan Tech 2003; 13(3): 141–8.
Obermeyer RJ, Fisher WE, Salameh JR, Jeyapalan M, Sweeney JF, Brunicardi FC: Laparoscopic pancreatic cystogastrostomy. Surg Laparosc Endosc Percutan Tech 2003; 13(4): 250–3.
Binmoeller KF, Jue P, Seifert H, Nam WC, Izbicki J, Soehendra N: Endoscopic pancreatic stent drainage in chronic pancreatitis and a dominant stricture: long-term results. Endoscopy 1995; 27(9): 638–44.
Baron TH, Harewood GC, Morgan DE, Yates MR: Outcome differences after endoscopic drainage of pancreatic necrosis, acute pancreatic pseudocysts, and chronic pancreatic pseudocysts. Gastrointest Endosc 2002; 56(1): 7–17.
Catalano MF, Geenen JE, Schmalz MJ, Johnson GK, Dean RS, Hogan WJ: Treatment of pancreatic pseudocysts with ductal communication by transpapillary pancreatic duct endoprosthesis. Gastrointest Endosc 1995; 42(3): 214–8.
Pitchumoni CS, Agarwal N: Pancreatic pseudocysts. When and how should drainage be performed? Gastroenterol Clin North Am 1999; 28(3): 615–39.
Grace PA, Williamson RC: Modern management of pancreatic pseudocysts. Br J Surg 1993; 80(5): 573–81.
Cantasdemir M, Kara B, Kantarci F, Mihmanli I, Numan F, Erguney S: Percutaneous drainage for treatment of infected pancreatic pseudocysts. South Med J 2003; 96(2): 136–40.
D'Egidio A, Schein M: Pancreatic pseudocysts: a proposed classification and its management implications. Br J Surg 1991; 78(8): 981–4.
Nealon WH, Walser E: Main pancreatic ductal anatomy can direct choice of modality for treating pancreatic pseudocysts (surgery versus percutaneous drainage). Ann Surg 2002; 235(6): 751–8.
Evans KA, Clark CW, Vogel SB, Behrns KE: Surgical management of failed endoscopic treatment of pancreatic disease. J Gastrointest Surg 2008; 12(11): 1924–9.
Martin RC, 2nd, Brennan MF, Jaques DP: Quality of complication reporting in the surgical literature. Ann Surg 2002; 235(6): 803–13.
Kooby DA, Stockman J, Ben-Porat L, Gonen M, Jarnagin WR, Dematteo RP, et al.: Influence of transfusions on perioperative and long-term outcome in patients following hepatic resection for colorectal metastases. Ann Surg 2003; 237(6): 860–9; discussion 869–70.
Traverso LW, Kozarek RA. Pancreatic necrosectomy: definitions and technique J Gastrointest Surg. 2005;9(3):436–9.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Boutros, C., Somasundar, P. & Espat, N.J. Open Cystogastrostomy, Retroperitoneal Drainage, and G-J Enteral Tube for Complex Pancreatitis-Associated Pseudocyst: 19 Patients with no Recurrence. J Gastrointest Surg 14, 1298–1303 (2010). https://doi.org/10.1007/s11605-010-1242-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-010-1242-7