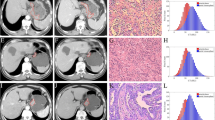
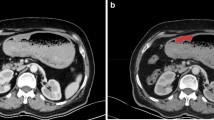
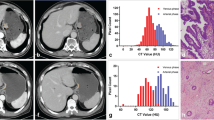
Abstract
Purpose
To examine the computed tomography (CT) images of patients with a diagnosis of gastric tumor by texture analysis and to investigate its place in differential diagnosis.
Materials and methods
Contrast enhanced venous phase CT images of 163 patients with pathological diagnosis of gastric adenocarcinoma (n = 125), gastric lymphoma (n = 12) and gastrointestinal stromal tumors (n = 26) were retrospectively analyzed. Pixel size adjustment, gray-level discretization and gray-level normalization procedures were applied as pre-processing steps. Region of interest (ROI) was determined from the axial slice that represented the largest lesion area and a total of 40 texture features were calculated for each patient. Texture features were compared between the tumor subtypes and between adenocarcinoma grades. Statistically significant texture features were combined into a single parameter by logistic regression analysis. The sensitivity and specificity of these features and the combined parameter were measured to differentiate tumor subtypes by receiver-operating characteristic curve (ROC) analysis.
Results
Classifications between adenocarcinoma versus lymphoma, adenocarcinoma vs. gastrointestinal stromal tumor (GIST) and well-differentiated adenocarcinoma versus poorly differentiated adenocarcinoma using texture features yielded successful results with high sensitivity (98, 91, 96%, respectively) and specificity (75, 77, 80%, respectively).
Conclusions
CT texture analysis is a non-invasive promising method for classifying gastric tumors and predicting gastric adenocarcinoma differentiation.






Similar content being viewed by others
References
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics [Erratum appears in CA Cancer J Clin. 2011 Mar–Apr;61(2):134]. CA Cancer J Clin. 2011;61:69–90.
Lee IS, Park YS, Lee JH, Park JY, Kim HS, Kim BS, et al. Pathologic discordance of differentiation between endoscopic biopsy and postoperative specimen in mucosal gastric adenocarcinomas. Ann Surg Oncol. 2013;20:4231–7.
Saito T, Kurokawa Y, Takiguchi S, Miyazaki Y, Takahashi T, Yamasaki M, et al. Accuracy of multidetector-row CT in diagnosing lymph node metastasis in patients with gastric cancer. Eur Radiol. 2015;25:368–74.
Tsurumaru D, Miyasaka M, Muraki T, Nishie A, Asayama Y, Oki E, et al. Histopathologic diversity of gastric cancers: relationship between enhancement pattern on dynamic contrast-enhanced CT and histological type. Eur J Radiol. 2017;97:90–5.
Tsurumaru D, Miyasaka M, Muraki T, Asayama Y, Nishie A, Oki E, et al. Diffuse-type gastric cancer: specific enhancement pattern on multiphasic contrast-enhanced computed tomography. Jpn J Radiol. 2017;35:289–95.
Zhao J, Ren G, Cai R, Chen J, Li H, Guo C, et al. Mucinous adenocarcinoma and non-mucinous adenocarcinoma: differing clinicopathological characteristics and computed tomography features in gastric cancer. Oncotarget. 2017;8:45698–709.
Hur BY, Kim SH, Choi JY, Rha SE, Lee MW, Kim SY, et al. Gastroduodenal glomus tumors: differentiation from other subepithelial lesions based on dynamic contrast-enhanced CT findings. Am J Roentgenol. 2011;197:1351–9.
Komori M, Asayama Y, Fujita N, Hiraka K, Tsurumaru D, Kakeji Y, et al. Extent of arterial tumor enhancement measured with preoperative mdct gastrography is a prognostic factor in advanced gastric cancer after curative resection. Am J Roentgenol. 2013;201:W253–61.
Tsurumaru D, Miyasaka M, Nishimuta Y, Asayama Y, Nishie A, Kawanami S, et al. Differentiation of early gastric cancer with ulceration and resectable advanced gastric cancer using multiphasic dynamic multidetector CT. Eur Radiol. 2016;26:1330–7.
Ma Z, Liang C, Huang Y, He L, Liang C, Chen X, et al. Can lymphovascular invasion be predicted by preoperative multiphasic dynamic CT in patients with advanced gastric cancer? Eur Radiol. 2017;27:3383–91.
Castellano G, Bonilha L, Li LM, Cendes F. Texture analysis of medical images. Clin Radiol. 2004;59:1061–9.
Bashir U, Siddique MM, McLean E, Goh V, Cook GJ. Imaging heterogeneity in lung cancer: techniques, applications, and challenges. Am J Roentgenol. 2016;207:534–43.
Gillies RJ, Kinahan PE, Hricak H. Radiomics: images are more than pictures, they are data. Radiology. 2016;278:563–77.
Aerts HJWL, Velazquez ER, Leijenaar RTH, Parmar C, Grossmann P, Cavalho S, et al. Decoding tumour phenotype by noninvasive imaging using a quantitative radiomics approach. Nat Commun. 2014;5:4006.
Schieda N, Lim RS, Krishna S, McInnes MDF, Flood TA, Thornhill RE. Diagnostic accuracy of unenhanced CT analysis to differentiate low-grade from high-grade chromophobe renal cell carcinoma. Am J Roentgenol. 2018;210:1079–87.
Davnall F, Yip CSP, Ljungqvist G, Selmi M, Ng F, Sanghera B, et al. Assessment of tumor heterogeneity: an emerging imaging tool for clinical practice? Insights Imaging. 2012;3:573–89.
Hodgdon T, McInnes MDF, Schieda N, Flood TA, Lamb L, Thornhill RE. Can quantitative CT texture analysis be used to differentiate fat-poor renal angiomyolipoma from renal cell carcinoma on unenhanced CT images? Radiology. 2015;276:787–96.
Liu S, Liu S, Ji C, Zheng H, Pan X, Zhang Y, et al. Application of CT texture analysis in predicting histopathological characteristics of gastric cancers. Eur Radiol. 2017;27:4951–9.
Giganti F, Antunes S, Salerno A, Ambrosi A, Marra P, Nicoletti R, et al. Gastric cancer: texture analysis from multidetector computed tomography as a potential preoperative prognostic biomarker. Eur Radiol. 2017;27:1831–9.
Nioche C, Orlhac F, Boughdad S, Reuze S, Goya-Outi J, Robert C, et al. Lifex: a freeware for radiomic feature calculation in multimodality imaging to accelerate advances in the characterization of tumor heterogeneity. Cancer Res. 2018;78:4786–9.
Kirienko M, Cozzi L, Antunovic L, Lozza L, Fogliata A, Voulaz E, et al. Prediction of disease-free survival by the PET/CT radiomic signature in non-small cell lung cancer patients undergoing surgery. Eur J Nucl Med Mol Imaging. 2018;45:207–17.
Yang J, Guo X, Ou X, Zhang W, Ma X. Discrimination of pancreatic serous cystadenomas from mucinous cystadenomas with CT textural features: based on machine learning. Front Oncol. 2019;9:494.
Larue RTHM, van Timmeren JE, de Jong EEC, Feliciani G, Leijenaar RTH, Schreurs WMJ, et al. Influence of gray level discretization on radiomic feature stability for different CT scanners, tube currents and slice thicknesses: a comprehensive phantom study. Acta Oncol (Madr). 2017;56:1544–53.
Collewet G, Strzelecki M, Mariette F. Influence of MRI acquisition protocols and image intensity normalization methods on texture classification. Magn Reson Imaging. 2004;22:81–91.
Shafiq-Ul-Hassan M, Zhang GG, Latifi K, Ullah G, Hunt DC, Balagurunathan Y, et al. Intrinsic dependencies of CT radiomic features on voxel size and number of gray levels. Med Phys. 2017;44:1050–62.
Ganeshan B, Burnand K, Young R, Chatwin C, Miles K. Dynamic contrast-enhanced texture analysis of the liver: initial assessment in colorectal cancer. Invest Radiol. 2011;46:160–8.
Lubner MG, Stabo N, Lubner SJ, del Rio AM, Song C, Halberg RB, et al. CT textural analysis of hepatic metastatic colorectal cancer: pre-treatment tumor heterogeneity correlates with pathology and clinical outcomes. Abdom Imaging. 2015;40:2331–7.
Antunovic L, De Sanctis R, Cozzi L, Kirienko M, Sagona A, Torrisi R, et al. PET/CT radiomics in breast cancer: promising tool for prediction of pathological response to neoadjuvant chemotherapy. Eur J Nucl Med Mol Imaging. 2019;46(7):1468–77.
Boellaard R, Delgado-Bolton R, Oyen WJG, Giammarile F, Tatsch K, Eschner W, et al. FDG PET/CT: EANM procedure guidelines for tumour imaging: version 2.0. Eur J Nucl Med Mol Imaging. 2015;42:328–54.
Ba-Ssalamah A, Muin D, Schernthaner R, Kulinna-Cosentini C, Bastati N, Stift J, et al. Texture-based classification of different gastric tumors at contrast-enhanced CT. Eur J Radiol. 2013. https://doi.org/10.1016/j.ejrad.2013.06.024.
Yan J, Chu-Shern JL, Loi HY, Khor LK, Sinha AK, Quek ST, et al. Impact of image reconstruction settings on texture features in 18F-FDG PET. J Nucl Med. 2015;56:1667–73.
Bailly C, Bodet-Milin C, Couespel S, Necib H, Kraeber-Bodéré F, Ansquer C, et al. Revisiting the robustness of PET-based textural features in the context of multi-centric trials. PLoS ONE. 2016. https://doi.org/10.1371/journal.pone.0159984.
Altazi BA, Zhang GG, Fernandez DC, Montejo ME, Hunt D, Werner J, et al. Reproducibility of F18-FDG PET radiomic features for different cervical tumor segmentation methods, gray-level discretization, and reconstruction algorithms. J Appl Clin Med Phys. 2017;18:32–48.
Yip SSF, Aerts HJWL. Applications and limitations of radiomics. Phys Med Biol. 2016;61:R150–66.
Ng F, Kozarski R, Ganeshan B, Goh V. Assessment of tumor heterogeneity by CT texture analysis: can the largest cross-sectional area be used as an alternative to whole tumor analysis? Eur J Radiol. 2013;82:342–8.
Ahn SJ, Kim JH, Park SJ, Han JK. Prediction of the therapeutic response after FOLFOX and FOLFIRI treatment for patients with liver metastasis from colorectal cancer using computerized CT texture analysis. Eur J Radiol. 2016;85:1867–74.
Funding
The authors received no financial support for the research, authorship, and publication of this article.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no financial interest in the materials presented herein.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Zeydanli, T., Kilic, H.K. Performance of quantitative CT texture analysis in differentiation of gastric tumors. Jpn J Radiol 40, 56–65 (2022). https://doi.org/10.1007/s11604-021-01181-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11604-021-01181-x