Abstract
Objective
Peritoneal fibrosis (PF) is the main cause of declining efficiency and ultrafiltration failure of the peritoneum, which restricts the long-term application of peritoneal dialysis (PD). This study aimed to investigate the therapeutic effects and mechanisms of bone marrow mesenchymal stem cells-derived exosomes (BMSC-Exos) on PF in response to PD.
Methods
Small RNA sequencing analysis of BMSC-Exos was performed by second-generation sequencing. C57BL/6J mice were infused with 4.25% glucose-based peritoneal dialysis fluid (PDF) for 6 consecutive weeks to establish a PF model. A total of 36 mice were randomly divided into 6 groups: control group, 1.5% PDF group, 2.5% PDF group, 4.25% PDF group, BMSC-Exos treatment group, and BMSC-Exos+TP53 treatment group. Reverse transcription quantitative polymerase chain reaction (RT-qPCR) was performed to measure the expression level of miR-27a-3p in BMSC-Exos and peritoneum of mice treated with different concentrations of PDF. HE and Masson staining were performed to evaluate the extent of PF. The therapeutic potential of BMSC-Exos for PF was examined through pathological examination, RT-qPCR, Western blotting, and peritoneal function analyses. Epithelial-mesenchymal transition (EMT) of HMrSV5 was induced with 4.25% PDF. Cells were divided into control group, 4.25% PDF group, BMSC-Exos treatment group, and BMSC-Exos+TP53 treatment group. Cell Counting Kit-8 assay was used to measure cell viability, and transwell migration assay was used to verify the capacity of BMSC-Exos to inhibit EMT in HMrSV5 cells.
Results
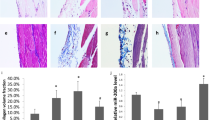
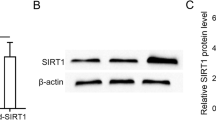
Small RNA sequencing analysis showed that miR-27a-3p was highly expressed in BMSC-derived exosomes compared to BMSCs. The RT-qPCR results showed that the expression of miR-27a-3p was upregulated in BMSC-Exos, but decreased in PD mice. We found that PF was glucose concentration-dependently enhanced in the peritoneum of the PD mice. Compared with the control mice, the PD mice showed high solute transport and decreased ultrafiltration volume as well as an obvious fibroproliferative response, with markedly increased peritoneal thickness and higher expression of α-SMA, collagen-I, fibronectin, and ECM1. The mice with PD showed decreased miR-27a-3p. Peritoneal structural and functional damage was significantly attenuated after BMSC-Exos treatment, while PF and mesothelial damage were significantly ameliorated. Additionally, markers of fibrosis (α-SMA, collagen-I, fibronectin, ECM1) and profibrotic cytokines (TGF-β1, PDGF) were downregulated at the mRNA and protein levels after BMSC-Exos treatment. In HMrSV5 cells, BMSC-Exos reversed the decrease in cell viability and the increase in cell migratory capacity caused by high-glucose PDF. Western blotting and RT-qPCR analysis revealed that BMSC-Exos treatment resulted in increased expression of E-cadherin (epithelial marker) and decreased expression of α-SMA, Snail, and vimentin (mesenchymal markers) compared to those of the 4.25% PDF-treated cells. Importantly, a dual-luciferase reporter assay showed that TP53 was a target gene of miR-27a-3p. TP53 overexpression significantly reversed the decreases in PF and EMT progression induced by BMSC-Exos.
Conclusion
The present results demonstrate that BMSC-Exos showed an obvious protective effect on PD-related PF and suggest that BMSC-derived exosomal miR-27a-3p may exert its inhibitory effect on PF and EMT progression by targeting TP53.
Similar content being viewed by others
References
Liyanage T, Ninomiya T, Jha V, et al. Worldwide access to treatment for end-stage kidney disease: a systematic review. Lancet, 2015,385(9981):1975–1982
Cho Y, Bello AK, Levin A, et al. Peritoneal Dialysis Use and Practice Patterns: An International Survey Study. Am J Kidney Dis, 2021,77(3):315–325
Holmes CJ, Faict D. Peritoneal dialysis solution biocompatibility: definitions and evaluation strategies. Kidney Int Suppl, 2003,(88):S50-S56
Krediet RT, Struijk DG. Peritoneal changes in patients on long-term peritoneal dialysis. Nat Rev Nephrol, 2013,9(7):419–429
Teitelbaum I. Ultrafiltration failure in peritoneal dialysis: a pathophysiologic approach. Blood Purif, 2015,39(1–3):70–73
Jagirdar RM, Bozikas A, Zarogiannis SG, et al. Encapsulating Peritoneal Sclerosis: Pathophysiology and Current Treatment Options. Int J Mol Sci, 2019,20(22):5765
Fan YP, Hsia CC, Tseng KW, et al. The Therapeutic Potential of Human Umbilical Mesenchymal Stem Cells From Wharton’s Jelly in the Treatment of Rat Peritoneal Dialysis-Induced Fibrosis. Stem Cells Transl Med, 2016,5(2):235–247
Ueno T, Nakashima A, Doi S, et al. Mesenchymal stem cells ameliorate experimental peritoneal fibrosis by suppressing inflammation and inhibiting TGF-beta1 signaling. Kidney Int, 2013,84(2):297–307
Nagasaki K, Nakashima A, Tamura R, et al. Mesenchymal stem cells cultured in serum-free medium ameliorate experimental peritoneal fibrosis. Stem Cell Res Ther, 2021,12(1):203
Wang J, Wang L, Xu L, et al. Targeting Src attenuates peritoneal fibrosis and inhibits the epithelial to mesenchymal transition. Oncotarget, 2017,8(48):83872–83889
Morishita Y, Ookawara S, Hirahara I, et al. HIF-1alpha mediates Hypoxia-induced epithelial-mesenchymal transition in peritoneal mesothelial cells. Ren Fail, 2016,38(2):282–289
Chen JS, Wong VW, Gurtner GC. Therapeutic potential of bone marrow-derived mesenchymal stem cells for cutaneous wound healing. Front Immunol, 2012,3:192
Han Y, Li X, Zhang Y, et al. Mesenchymal Stem Cells for Regenerative Medicine. Cells, 2019,8(8):886
Nagaishi K, Mizue Y, Chikenji T, et al. Mesenchymal stem cell therapy ameliorates diabetic nephropathy via the paracrine effect of renal trophic factors including exosomes. Sci Rep, 2016,6:34842
Li M, Li S, Du C, et al. Exosomes from different cells: Characteristics, modifications, and therapeutic applications. Eur J Med Chem, 2020,207:112784
Murray L, Krasnodembskaya AD. Concise Review: Intercellular Communication Via Organelle Transfer in the Biology and Therapeutic Applications of Stem Cells. Stem Cells, 2019,37(1):14–25
Fan C, Wang Q, Chen Y, et al. Exosomes derived from bone mesenchymal stem cells attenuate myocardial fibrosis both in vivo and in vitro via autophagy activation: the key role of miR-199a-3p/mTOR pathway. Hum Cell, 2022,35(3):817–835
Ma J, Li Y, Chen M, et al. hMSCs-derived exosome circCDK13 inhibits liver fibrosis by regulating the expression of MFGE8 through miR-17-5p/KAT2B. Cell Biol Toxicol, 2023,39(2):1–22
Sun C, Shi C, Duan X, et al. Exosomal microRNA-618 derived from mesenchymal stem cells attenuate the progression of hepatic fibrosis by targeting Smad4. Bioengineered, 2022,13(3):5915–5927
Xu L, Fan Y, Wu L, et al. Exosomes from Bone Marrow Mesenchymal Stem Cells with Overexpressed Nrf2 Inhibit Cardiac Fibrosis in Rats with Atrial Fibrillation. Cardiovasc Ther, 2022,2022:2687807
Yao L, Ye Y, Mao H, et al. MicroRNA-124 regulates the expression of MEKK3 in the inflammatory pathogenesis of Parkinson’s disease. J Neuroinflammation, 2018,15(1):13
Hu J, Shan Z, Hu K, et al. miRNA-223 inhibits epithelial-mesenchymal transition in gastric carcinoma cells via Sp1. Int J Oncol, 2016,49(1):325–335
Jaca A, Govender P, Locketz M, et al. The role of miRNA-21 and epithelial mesenchymal transition (EMT) process in colorectal cancer. J Clin Pathol, 2017,70(4):331–356
Domingues C, Serambeque BP, Laranjo CM, et al. Epithelial-mesenchymal transition and microRNAs: Challenges and future perspectives in oral cancer. Head Neck, 2018,40(10):2304–2313.
Lin F, Wu X, Zhang H, et al. A microrna screen to identify regulators of peritoneal fibrosis in a rat model of peritoneal dialysis. BMC Nephrol, 2015,16:48
Li X, Liu H, Sun L, et al. MicroRNA-302c modulates peritoneal dialysis-associated fibrosis by targeting connective tissue growth factor. J Cell Mol Med, 2019,23(4):2372–2383
Szeto CC, Chow KM, Kwan BC, et al. Peritoneal dialysis effluent miR-21 and miR-589 levels correlate with longitudinal change in peritoneal transport characteristics. Clin Chim Acta, 2017,464:106–112
Xi Y, Shen Y, Wu D, et al. CircBCAR3 accelerates esophageal cancer tumorigenesis and metastasis via sponging miR-27a-3p. Mol Cancer, 2022,21(1):145
Guo D, Li Y, Chen Y, et al. DANCR promotes HCC progression and regulates EMT by sponging miR-27a-3p via ROCK1/LIMK1/COFILIN1 pathway. Cell Prolif, 2019,52(4):e12628
Bello A K, Okpechi I G, Osman M A, et al. Epidemiology of peritoneal dialysis outcomes. Nat Rev Nephrol, 2022,18(12):779–793
Gu C, Feng J, Waqas A, et al. Technological Advances of 3D Scaffold-Based Stem Cell/Exosome Therapy in Tissues and Organs. Front Cell Dev Biol, 2021,9:709204
Fang Y, Garnier D, Lee TH, et al. PML-RARa modulates the vascular signature of extracellular vesicles released by acute promyelocytic leukemia cells. Angiogenesis, 2016,19(1):25–38
Vanherle S, Haidar M, Irobi J, et al. Extracellular vesicle-associated lipids in central nervous system disorders. Adv Drug Deliv Rev, 2020,159:322–331
Sabaratnam R, Geertsen L, Skjodt K, et al. In human nephrectomy specimens, the kidney level of tubular transport proteins does not correlate with their abundance in urinary extracellular vesicles. Am J Physiol Renal Physiol, 2019,317(3):F560–F571
Krediet R T. Ultrafiltration Failure Is a Reflection of Peritoneal Alterations in Patients Treated With Peritoneal Dialysis. Front Physiol, 2018,9:1815
Yanai K, Ishii H, Aomatsu A, et al. MicroRNAs in peritoneal fibrosis: a systematic review. Discov Med, 2018,26(145):271–280
Shin HS, Ko J, Kim DA, et al. Metformin ameliorates the Phenotype Transition of Peritoneal Mesothelial Cells and Peritoneal Fibrosis via a modulation of Oxidative Stress. Sci Rep, 2017,7(1):5690
Ji S, Deng H, Jin W, et al. Beta-catenin participates in dialysate-induced peritoneal fibrosis via enhanced peritoneal cell epithelial-to-mesenchymal transition. FEBS Open Bio, 2017,7(2):265–273
Sung SA, Kim DH, Oh KH, et al. The Role of Cathepsin B in Peritoneal Fibrosis due to Peritoneal Dialysis. Int J Nephrol, 2019,2019:4150656
Yang A H, Chen JY, Lin JK. Myofibroblastic conversion of mesothelial cells. Kidney Int, 2003,63(4):1530–1539
Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest, 2009,119(6):1420–1428
Zhao JL, Guo MZ, Zhu JJ, et al. Curcumin suppresses epithelial-to-mesenchymal transition of peritoneal mesothelial cells (HMrSV5) through regulation of transforming growth factor-activated kinase 1 (TAK1). Cell Mol Biol Lett, 2019,24:32
Haywood ME, Cocciolo A, Porter KF, et al. Transcriptome signature of ventricular arrhythmia in dilated cardiomyopathy reveals increased fibrosis and activated TP53. J Mol Cell Cardiol, 2020,139:124–134
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that there is no conflict of interest with any financial organization or corporation or individual that can inappropriately influence this work.
Additional information
This study was supported by the Technology Development Program of Shanghai Pudong New District (No. PKJ2021-Y34) and the Excellent Young Medical Talent Training Program of Pudong Health Commission of Shanghai (No. PWRq2022-18).
Rights and permissions
About this article
Cite this article
Zhao, Jl., Zhao, L., Zhan, Qn. et al. BMSC-derived Exosomes Ameliorate Peritoneal Dialysis-associated Peritoneal Fibrosis via the Mir-27a-3p/TP53 Pathway. CURR MED SCI 44, 333–345 (2024). https://doi.org/10.1007/s11596-024-2853-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11596-024-2853-7