Abstract
Objective
Diabetic nephropathy is one of the most important microvascular complications of diabetes, which mainly refers to glomerular capillary sclerosis. Podocytes are an important part of glomerular capillaries. Previous clinical and basic studies have shown that fibrosis is the main factor of diabetic nephropathy. This study aimed to assess the protective mechanism of glycyrrhizic acid (GA) on glomerular podocytes induced by high glucose as we hypothesized that GA may have antifibrotic and anti-inflammatory effects on podocytes through regulation of the adenosine 5′-monophosphate-activated protein kinase (AMPK)/sucrose nonfermenting AMPK-related kinase (SNARK) signaling pathway.
Methods
SNARK siRNA was used to transfect podocytes. Real-time quantitative polymerase chain reaction and immunofluorescence staining assays were used for molecular and pathological analysis. The expression levels of key pathway proteins (including TGF-β1, α-SMA, SITR1, AMPKα, LKB1, PGC-1α, NF-κB, IL-6, and TNF-α) were verified by Western blotting. The expression of inflammatory factors in podocytes was detected by ELISA.
Results
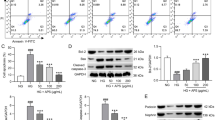
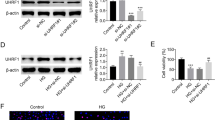
We demonstrated that GA decreased the expression of podocyte fibrosis signaling pathway-related factors by upregulating the AMPK pathway and its related factors. However, after transfection of podocytes with SNARK siRNA, there was an increased expression of fibrosis-related factors and inflammation-related factors.
Conclusion
GA can protect podocytes and alleviate fibrosis and inflammation induced by high glucose, which is related to the AMPK signaling pathway. Meanwhile, knockdown of SNARK protein can inhibit the AMPK signaling pathway, aggravate fibrosis, and increase inflammation.
Similar content being viewed by others
References
Tsoutsouki J, Wunna W, Chowdhury A, et al. Advances in the management of diabetes: therapies for type 2 diabetes. Postgrad Med J, 2020,96(1140):610–618
Libianto R, Davis TM, Ekinci EI, et al. Advances in type 2 diabetes therapy: a focus on cardiovascular and renal outcomes. Med J Aust, 2020,212(3):133–139
Horikoshi S, Fukuda N, Tsunemi A, et al. Contribution of TGF-beta1 and Effects of Gene Silencer Pyrrole-Imidazole Polyamides Targeting TGF-beta1 in Diabetic Nephropathy. Molecules, 2020,25(4):950
Chung JY, Chan MK, Li JS, et al. TGF-beta Signaling: From Tissue Fibrosis to Tumor Microenvironment. Int J Mol Sci, 2021,22(14):7575
Wang Y, Jia L, Hu Z, et al. AMP-activated protein kinase/myocardin-related transcription factor-A signaling regulates fibroblast activation and renal fibrosis. Kidney Int, 2018,93(1):81–94
Coughlan KA, Valentine RJ, Ruderman NB, et al. AMPK activation: a therapeutic target for type 2 diabetes? Diabetes Metab Syndr Obes, 2014,7:241–253
Li NS, Zou JR, Lin H, et al. LKB1/AMPK inhibits TGF-beta1 production and the TGF-beta signaling pathway in breast cancer cells. Tumour Biol, 2016,37(6):8249–8258
Lefebvre DL, Bai Y, Shahmolky N, et al. Identification and characterization of a novel sucrose-non-fermenting protein kinase/AMP-activated protein kinase-related protein kinase, SNARK. Biochem J, 2001,355(2):297–305
Goto K, Kato N, Chung RT, et al. Anti-hepatocellular carcinoma properties of the anti-alcoholism drug disulfiram discovered to enzymatically inhibit the AMPK-related kinase SNARK in vitro. Oncotarget, 2016,7(46):74987–74999
Courchet J, Lewis TL, Lee S, et al. Terminal axon branching is regulated by the LKB1-NUAK1 kinase pathway via presynaptic mitochondrial capture. Cell, 2013,153(7):1510–1525
Sun XL, Lessard SJ, An D, et al. Sucrose nonfermenting AMPK-related kinase (SNARK) regulates exercise-stimulated and ischemia-stimulated glucose transport in the heart. J Cell Biochem, 2019,120(1):685–696
Liu B, Gan X, Zhao Y, et al. Inhibition of HMGB1 reduced high glucose-induced BMSCs apoptosis via activation of AMPK and regulation of mitochondrial functions. J Physiol Biochem, 2021,77(2):227–235
Liu Y. New insights into epithelial-mesenchymal transition in kidney fibrosis. J Am Soc Nephrol, 2010,21(2):212–222
Tang Q, Cao Y, Xiong W, et al. Glycyrrhizic acid exerts protective effects against hypoxia/reoxygenation-induced human coronary artery endothelial cell damage by regulating mitochondria. Exp Ther Med, 2020,20(1):335–342
Wang YJ, Wang Z, Zhao TQ, et al. Effect of Glycyrrhizic Acid on High Glucose Induced Podocyte Injury in Mice. J Ningxia Med Univ (Chinese), 2022,44(03):267–271
Rajab BS, Albukhari TA, Khan AA, et al. Antioxidative and Anti-Inflammatory Protective Effects of beta-Caryophyllene against Amikacin-Induced Nephrotoxicity in Rat by Regulating the Nrf2/AMPK/AKT and NF-kappaB/TGF-beta/KIM-1 Molecular Pathways. Oxid Med Cell Longev, 2022,2022:4212331
Zhang YL, Li PB, Han X, et al. Blockage of Fibronectin 1 Ameliorates Myocardial Ischemia/Reperfusion Injury in Association with Activation of AMP-LKB1-AMPK Signaling Pathway. Oxid Med Cell Longev, 2022,2022:6196173
Umanath K, Lewis JB. Update on Diabetic Nephropathy: Core Curriculum. Am J Kidney Dis, 2018,71(6):884–895
Bhatt DL, Szarek M, Pitt B, et al. Sotagliflozin in Patients with Diabetes and Chronic Kidney Disease. N Engl J Med, 2021,384(2):129–139
Chen J, Chen JK, Harris RC, et al. EGF receptor deletion in podocytes attenuates diabetic nephropathy. J Am Soc Nephrol, 2015,26(5):1115–1125
Wu M, Yang Z, Zhang C, et al. Inhibition of NLRP3 inflammasome ameliorates podocyte damage by suppressing lipid accumulation in diabetic nephropathy. Metabolism, 2021,118:154748
Song S, Qiu D, Shi Y, et al. Thioredoxin-interacting protein deficiency alleviates phenotypic alterations of podocytes via inhibition of mTOR activation in diabetic nephropathy. J Cell Physiol, 2019,234(9):16485–16502
Gil CL, Hooker E, Larrivee B, et al. Diabetic Kidney Disease, Endothelial Damage, and Podocyte-Endothelial Crosstalk. Kidney Med, 2020,3(1):105–115
Guo Q, Zhong W, Duan A, et al. Protective or deleterious role of Wnt/beta-catenin signaling in diabetic nephropathy: An unresolved issue. Pharmacol Res, 2019,144:151–157
Chaudhuri A, Ghanim H, Arora P, et al. Improving the residual risk of renal and cardiovascular outcomes in diabetic kidney disease: A review of pathophysiology, mechanisms, and evidence from recent trials. Diabetes Obes Metab, 2022,24(3):365–376
Grahammer F, Schell C, Huber TB, et al. The podocyte slit diaphragm—from a thin grey line to a complex signalling hub. Nat Rev Nephrol, 2013,9(10):587–598
Wang D, Sant S, Ferrell N, et al. A Biomimetic In Vitro Model of the Kidney Filtration Barrier Using Tissue-Derived Glomerular Basement Membrane. Adv Healthc Mater, 2021,10(16):e2002275
Yoshida S, Wei X, Zhang G, et al. Endoplasmic reticulum-associated degradation is required for nephrin maturation and kidney glomerular filtration function. J Clin Invest, 2021,131(7):e143988
Lu Q, Hou Q, Cao K, et al. Complement factor B in high glucose-induced podocyte injury and diabetic kidney disease. JCI Insight, 2021,6(19):e147716
Lin CL, Hsu YC, Huang YT, et al. A KDM6A-KLF10 reinforcing feedback mechanism aggravates diabetic podocyte dysfunction. EMBO Mol Med, 2019,11(5):e9828
Sheng L, Zhuang S. New Insights Into the Role and Mechanism of Partial Epithelial-Mesenchymal Transition in Kidney Fibrosis. Front Physiol, 2020,11:569322
Li L, Feng Y, Zhang J, et al. Microtubule associated protein 4 phosphorylation-induced epithelial-to-mesenchymal transition of podocyte leads to proteinuria in diabetic nephropathy. Cell Commun Signal, 2022,20(1):115
Cherney DZI, Dekkers CCJ, Barbour SJ, et al. Effects of the SGLT2 inhibitor dapagliflozin on proteinuria in non-diabetic patients with chronic kidney disease (DIAMOND): a randomised, double-blind, crossover trial. Lancet Diabetes Endocrinol, 2020,8(7):582–593
Perkovic V, Jardine MJ, Neal B, et al. Canagliflozin and Renal Outcomes in Type 2 Diabetes and Nephropathy. N Engl J Med, 2019,380(24):2295–2306
Gujarati NA, Leonardo AR, Vasquez JM, et al. Loss of Functional SCO2 Attenuates Oxidative Stress in Diabetic Kidney Disease. Diabetes, 2021,db210316
Galvan DL, Long J, Green N, et al. Drp1S600 phosphorylation regulates mitochondrial fission and progression of nephropathy in diabetic mice. J Clin Invest, 2019,129(7):2807–2823
Sun W, Wang Y, Zheng Y, et al. The Emerging Role of Sestrin2 in Cell Metabolism, and Cardiovascular and Age-Related Diseases. Aging Dis, 2020,11(1):154–163
Juszczak F, Caron N, Mathew AV, et al. Critical Role for AMPK in Metabolic Disease-Induced Chronic Kidney Disease. Int J Mol Sci, 2020,21(21):7994
Song S, Shi C, Bian Y, et al. Sestrin2 remedies podocyte injury via orchestrating TSP-1/TGF-beta1/Smad3 axis in diabetic kidney disease. Cell Death Dis, 2022,13(7):663
Wu M, Chen G, Li YP, et al. TGF-beta and BMP signaling in osteoblast, skeletal development, and bone formation, homeostasis and disease. Bone Res, 2016,4:16009
Zhang YD, Zhao SC, Zhu ZS, et al. Cx43- and Smad-Mediated TGF-beta/ BMP Signaling Pathway Promotes Cartilage Differentiation of Bone Marrow Mesenchymal Stem Cells and Inhibits Osteoblast Differentiation. Cell Physiol Biochem, 2017,42(4):1277–1293
Li J, Li N, Yan S, et al. Melatonin attenuates renal fibrosis in diabetic mice by activating the AMPK/PGC1alpha signaling pathway and rescuing mitochondrial function. Mol Med Rep, 2019,19(2):1318–1330
Kim H, Moon SY, Kim JS, et al. Activation of AMP-activated protein kinase inhibits ER stress and renal fibrosis. Am J Physiol Renal Physiol, 2015,308(3):F226–F236
Gamad N, Malik S, Suchal K, et al. Metformin alleviates bleomycin-induced pulmonary fibrosis in rats: Pharmacological effects and molecular mechanisms. Biomed Pharmacother, 2018,97:1544–1553
Wang L, Tian Y, Shang Z, et al. Metformin attenuates the epithelial-mesenchymal transition of lens epithelial cells through the AMPK/TGF-beta/Smad2/3 signalling pathway. Exp Eye Res, 2021,212:108763
van de Vis RAJ, Moustakas A, van der Heide LP, et al. NUAK1 and NUAK2 Fine-Tune TGF-beta Signaling. Cancers (Basel), 2021,13(13):3377
Kolliopoulos C, Raja E, Razmara M, et al. Transforming growth factor beta (TGFbeta) induces NUAK kinase expression to fine-tune its signaling output. J Biol Chem, 2019,294(11):4119–4136
Feng X, Chen X, Zaeem M, et al. Sesamol Attenuates Neuroinflammation by Regulating the AMPK/SIRT1/NF-kappaB Signaling Pathway after Spinal Cord Injury in Mice. Oxid Med Cell Longev, 2022,2022:8010670
Xu T, Wang S, Li X, et al. Lithium chloride represses abdominal aortic aneurysm via regulating GSK3beta/SIRT1/NF-kappaB signaling pathway. Free Radic Biol Med, 2021,166:1–10
Gao C, Fei X, Wang M, et al. Cardamomin protects from diabetes-induced kidney damage through modulating PI3K/AKT and JAK/STAT signaling pathways in rats. Int Immunopharmacol, 2022,107:108610
Sun HJ, Xiong SP, Cao X, et al. Polysulfide-mediated sulfhydration of SIRT1 prevents diabetic nephropathy by suppressing phosphorylation and acetylation of p65 NF-kappaB and STAT3. Redox Biol, 2021,38:101813
Kimura Y, Yanagida T, Onda A, et al. Soluble Uric Acid Promotes Atherosclerosis via AMPK (AMP-Activated Protein Kinase)-Mediated Inflammation. Arterioscler Thromb Vasc Biol, 2020,40(3):570–582
Kato K, Tokuda H, Matsushima-Nishiwaki R, et al. AMPK limits IL-1-stimulated IL-6 synthesis in osteoblasts: involvement of IkappaB/NF-kappaB pathway. Cell Signal, 2012,24(8):1706–1712
Oh H, Park SH, Kang MK, et al. Asaronic Acid Attenuates Macrophage Activation toward M1 Phenotype through Inhibition of NF-kappaB Pathway and JAK-STAT Signaling in Glucose-Loaded Murine Macrophages. J Agric Food Chem, 2019,67(36):10069–10078
Cao XJ, Wu R, Qian HY, et al. Metformin attenuates diabetic neuropathic pain via AMPK/NF-kappaB signaling pathway in dorsal root ganglion of diabetic rats. Brain Res, 2021,1772:147663
Li F, Chen Y, Li Y, et al. Geniposide alleviates diabetic nephropathy of mice through AMPK/SIRT1/NF-kappaB pathway. Eur J Pharmacol, 2020,886:173449
Mancini SJ, White AD, Bijland S, et al. Activation of AMP-activated protein kinase rapidly suppresses multiple pro-inflammatory pathways in adipocytes including IL-1 receptor-associated kinase-4 phosphorylation. Mol Cell Endocrinol, 2017,440:44–56
Huang W, Shang WL, Wang HD, et al. Sirt1 overexpression protects murine osteoblasts against TNF-alpha-induced injury in vitro by suppressing the NF-kappaB signaling pathway. Acta Pharmacol Sin, 2012,33(5):668–674
Lee IY, Lim JM, Cho H, et al. MST1 Negatively Regulates TNFalpha-Induced NF-kappaB Signaling through Modulating LUBAC Activity. Mol Cell, 2019,73(6):1138–1149.e6
Li HN, Yang QQ, Wang WT, et al. Red nucleus IL-33 facilitates the early development of mononeuropathic pain in male rats by inducing TNF-alpha through activating ERK, p38 MAPK, and JAK2/STAT3. J Neuroinflammation, 2021,18(1):150
Fadaei R, Bagheri N, Heidarian E, et al. Serum levels of IL-32 in patients with type 2 diabetes mellitus and its relationship with TNF-alpha and IL-6. Cytokine, 2020,125:154832
Pang R, Gu D. Triptolide Improves Renal Injury in Diabetic Nephropathy Rats through TGF-beta1/Smads Signal Pathway. Endocr Metab Immune Disord Drug Targets, 2021,21(10):1905–1911
Zitman-Gal T, Einbinder Y, Ohana M, et al. Effect of liraglutide on the Janus kinase/signal transducer and transcription activator (JAK/STAT) pathway in diabetic kidney disease in db/db mice and in cultured endothelial cells. J Diabetes, 2019,11(8):656–664
Johnson DE, O’Keefe RA, Grandis JR, et al. Targeting the IL-6/JAK/STAT3 signalling axis in cancer. Nat Rev Clin Oncol, 2018,15(4):234–248
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Additional information
This work was supported by the Natural Science Foundation of Ningxia Province (No. 2021AAC03296).
Rights and permissions
About this article
Cite this article
Zhao, Tq., Li, Y., Zhang, M. et al. Glycyrrhizic Acid Protects Glomerular Podocytes Induced by High Glucose by Modulating SNARK/AMPK Signaling Pathway. CURR MED SCI 43, 696–707 (2023). https://doi.org/10.1007/s11596-023-2765-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11596-023-2765-y